Have you ever wondered how plants are able to absorb water from the soil and transport it to their leaves? Or how the cells in our bodies are able to maintain the proper balance of fluids? All of these processes hinge on a single phenomenon called osmosis.
Osmosis is a biophysical phenomenon in which water (or another solvent) moves from a less concentrated solution to a more concentrated solution through a partially permeable membrane, which lets some particles pass while blocking others. This movement is also sometimes referred to as “down the concentration gradient”.
The solvent will maintain this migration until equilibrium in concentration is reached.
Imagine two cups of water, one with a high concentration of salt and the other with a low concentration. If you were to place a semi-permeable membrane between them, the water from the low-concentration cup would flow into the high-concentration cup until the concentration on both sides is equal.

Osmotic pressure is the force required to prevent water movement across the semipermeable membrane.
The term osmosis, which is Greek for ‘thrust’ or ‘impulse’, was first coined by J.A. Nollet, who in 1747 described an experiment in which he used an animal bladder to separate two chambers containing water and wine. He noticed that the volume in the chamber containing wine increased and, if the chamber was closed, pressure rose.
How osmosis works
A classic experiment for osmosis involves splitting a beaker of water into two halves, with a semipermeable membrane in between and salt added to one of the sides. You’ll soon notice water migrating from the side of the beaker with no salt at all to the side with the saline solution. This movement of water will continue until the concentration of salt is the same on both sides.
It’s the same reason why you should never put a snail near salt, which would cause the poor creature to die as its water is extracted from it.
Key to osmosis is the presence of a semipermeable membrane that makes it more likely for water molecules in a low-concentration solution to collide with the membrane and pass through, whereas water molecules in a concentrated solution will have far fewer molecules of water colliding with the membrane and passing through. This mismatch means that there’s a greater statistical probability of more water molecules passing through the membrane from a less concentrated solution. Once the statistical probability of water molecules passing through the membrane is equal, osmosis stops.
Osmosis in nature
Osmosis is one of the essential processes of life. Each cell of our body, as well as those of plants and animals, owe their survival to osmosis.
Take plants, for instance. When we water them, we pour water on the stem end and soil. If the plant’s cells are surrounded by a solution that contains a higher concentration of water molecules (low solute concentration) than the solution inside the cells, water will enter the leaves, fruits, and flowers by osmosis. During this process, the plant cell will become firm.
However, if a plant is surrounded by a solution that contains a lower concentration of water (higher solute concentration), then the water molecules of the solution inside the plant’s cells will be expelled by osmosis, turning the plant flaccid.
The roots of a plant have semi-permeable membranes that allow water to flow in while keeping out larger particles, such as soil. The water then travels up the stem and into the leaves through small vessels called xylem.
Once in the leaves, the water is used in the process of photosynthesis, which converts sunlight into energy for the plant. Any excess water is then released through small openings called stomata. This process is known as transpiration and helps to regulate the temperature and humidity of the surrounding environment.
Perhaps another more relatable example of osmosis at work is found within our own bodies. When we drink water, cells absorb it by osmosis just like plant roots. The cell wall acts as a semipermeable membrane, creating osmotic pressure between the inside and outside of the cell. Blood is a more dilute solution than the cell’s cytoplasm, so water from the blood will cross through the cell wall. The same applies to nutrients and minerals, which are also transferred by osmosis.
When the concentration of electrolytes, such as sodium or potassium, on one side of the membrane is higher than the other, water flows in or out of the cell in order to balance the concentrations. This process is essential for maintaining proper cell function and overall health.
Humans have recognized the potential of osmosis since antiquity, employing it to preserve foods. The ancients observed that adding salt or sugar removes water from tissues. At the time, the process was called imbibition due to the fact that solutes like salt and sugar attracted the water from the material they touched.
Today, osmosis is important for a number of different industries. The process of reverse osmosis is widely used in the treatment of water, particularly in desalination plants, where seawater is forced through a semi-permeable membrane, which separates the salt and other dissolved minerals from the water. This process, known as reverse osmosis, is able to remove up to 99% of dissolved salt, making the water safe for drinking and irrigation.
What is reverse osmosis?
Reverse osmosis, as the name suggests, is the reverse of osmosis. Instead of a solvent, such as water, flowing from a less concentrated solution to a more concentrated one, it flows in the opposite direction, through a semi-permeable membrane, from a more concentrated solution to a less concentrated one. This happens when pressure is applied on one side of the membrane.
Reverse osmosis is typically used in water purification systems, where the goal is to remove impurities such as bacteria, viruses, and dissolved solids like salt, fluoride, and heavy metals. The process begins by pressurizing the water, forcing it through a semipermeable membrane. As the water flows through the membrane, impurities are left behind, creating a purified stream of water on the other side.
Reverse osmosis systems can vary in design and complexity, but typically consist of several key components. These include a pre-filter, which removes larger particles such as sediment and dirt, a high-pressure pump, which increases the water pressure to force it through the membrane, and the semipermeable membrane itself, which is responsible for removing impurities.
One of the most notable applications of reverse osmosis is the desalination of seawater. With the world’s population continuing to grow, and freshwater resources becoming increasingly scarce, desalination is becoming an increasingly important solution for providing clean drinking water.
Reverse osmosis desalination plants work by pressurizing seawater and forcing it through a semi-permeable membrane. As the seawater flows through the membrane, salt, and other impurities are left behind, creating a stream of freshwater. It is estimated that reverse osmosis desalination plants can produce freshwater at a cost of around $0.50 to $1.00 per cubic meter.
What’s the difference between osmosis and diffusion?
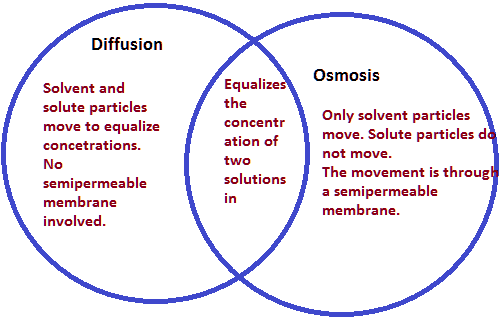
Diffusion is the general movement of any type of molecule or particle from an area of higher concentration to an area of lower concentration. Diffusion and osmosis are both passive transport processes, meaning they require no energy input to move substances. Both processes are essential to the proper functioning of biological processes such as the transport of water or nutrients between cells.
The main difference between the two is that diffusion can occur in any mixture, even when two solutions aren’t separated by a semipermeable membrane, whereas osmosis exclusively occurs across a semipermeable membrane.
Diffusion makes air composition uniform by redistributing chemical species, such as oxygen in the air until equilibrium is reached: in other words, until the concentration gradient — the difference in concentration between two areas — has been eliminated. If the concentration of a species is not initially uniform, over time, diffusion will cause a mass transfer in favor of a more uniform concentration.
| Osmosis | Diffusion |
|---|---|
| Involves the movement of a solvent across a semi-permeable membrane. | Involves the movement of any type of molecule or particle, regardless of its size or charge. |
| The solvent is usually water. | The molecule or particle can be any substance. |
| The movement is driven by a difference in the concentration of the solvent. | The movement is driven by a difference in concentration of any type of molecule or particle. |
| Osmotic pressure is generated when the solvent moves across the membrane. | No pressure is generated in diffusion. |
| Occurs in both plant and animal cells. | Occurs in both living and non-living systems. |
Bottom line: osmosis — the natural movement of water into a solution through a semipermeable membrane — is central to all of biology. It is a passive transport process like diffusion, but the two are distinct.