Each one of us has defining features, things that make us undeniably ourselves. Atoms are the same. This it is a property known as their ‘atomic number’, and it represents the number of protons at their core.

The world around us is made of a large variety of chemical substances and almost all of them are mixtures. The air we breathe, for example, is a cocktail of gases. A slice of apple pie is an incredibly complex tapestry of chemicals. And for all of these cocktails and mixtures, the base components that make them up are chemical elements.
Each element is a single type of atom. What makes them unique, distinct elements is the particular way each interacts with the substances around them. And this behavior, in turn, is caused by their particular structure. The atomic number describes the most important element of this atomic structure, making it, essentially, the calling card of each particular element.
Let’s see why.
The atomic calling card
Atoms are tiny round things that make everything up. They, themselves, are constructed from subatomic particles, namely protons, electrons, and neutrons. Neutrons stand out among these three as they don’t really do much inside the atom; they just weigh it down while not having any meaningful input on how the atom interacts with the world around it.
The atomic number, or ‘Z’, records the number of protons at an atom’s core. This is a stable and reliable quantity, whereas the number of electrons and neutrons inside an atom can vary (which produce ions and isotopes, respectively). Because of the very tight correlation between Z and the number of protons in an atom’s core, it is also known as an element’s ‘proton number’.
Protons are (positively) charged particles; as such, they attract negatively charged particles in the shape of electrons to orbit around them. In atoms with neutral electric charge (a state of balance that all atoms try to reach), each proton in the core will have its own corresponding electron zipping around in the atom’s shell such that the charges balance each other out.
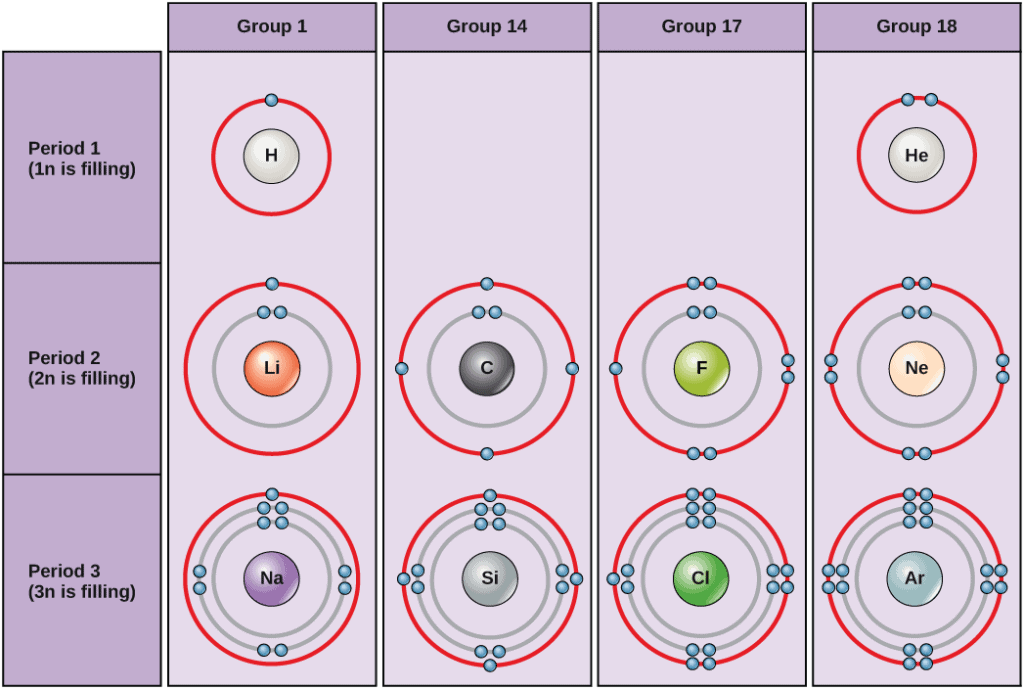
The behavior of the electrons in an atom’s shell gives rise to much of their chemical properties. As such, the atomic number gives us a quick description of the way individual chemical elements behave in nature — especially in regards to how readily they will bind with other elements. This means that if you somehow change an atom’s number, its behavior, properties, and the way it interacts with its environment change as well — in essence, it becomes a whole new element.
If we take all this together, what we are left with is this: the atoms of each chemical element are characterized by a single atomic number. Each element has its own atomic number and only atoms of that element have that atomic number. It tells us how many protons we’ll find at their core, which has a huge influence on the atom’s chemical properties.
How did we get it?
The periodic table of the elements as we know it today was first created by Russian chemist Dmitri Mendeleev around 1869. It denotes all of the elements known to man, both the natural ones and those we’ve generated in the lab. Each element is represented in a square block with its symbol (made up of letters), with two numbers: the one on the top is its atomic number, while the one on the bottom is its atomic weight (the weight of protons and neutrons put together).
All the elements in the periodic table are ordered progressively by their atomic number. Hydrogen, the first one in the table, has an atomic number of 1. It is followed by helium, with an atomic number of 2, lithium with an atomic number of 3, and so on.
Although its shape has not been altered meaningfully since its earliest days, Mendeleev didn’t actually use the atomic number to order elements in his new table. Scientists at the time were not aware of the existence of protons, and as such were completely incapable of measuring an element’s atomic number. But they were able to measure an element’s atomic weight; the earliest drafts of the table were put together starting from atomic weights and tweaked in places based on the observable properties of different elements. For example, Mendeleev placed tellurium (atomic weight 127.6) ahead of iodine (atomic weight 126.9) based on the chemicals’ behavior.
To simplify everything, each atom was given a number to signify its place in the new table — a ‘Zahl‘ in German. Hydrogen would get number 1, helium number 2, and so forth.
This simple table would cause a paradigm shift in chemistry. It proved so reliable that Mendeleev was actually able to estimate the existence of chemical elements which were not yet known at the time based on which spots were ‘open’ in the table. The structure of the table also suggested the existence of groups of elements with similar properties, allowing him to also estimate the properties of those yet-unknown elements, giving researchers hints on how to best isolate them.
Being able to draw such a wealth of information hinted at the fact that the table, without Mendeleev’s knowledge, was based on something more than just each element’s weight; around 1913, researchers finally got their first look at what that something was.
After researchers started mapping the internal structures of atoms, amateur Dutch physicist Antonius van den Broek proposed that it is actually the electrical charge contained in each atom’s core that gives it its position in the periodic table. The term “atomic number” was adopted to refer to this charge and, in 1914, the hypothesis was also confirmed experimentally. An atom’s nuclear charge is produced by the number of protons at its core and as such, this charge is identical to its atomic number.
These breakthroughs in 1913 and 1914 set the stage for researchers to really peer into the structure and physics of the atom. They formed the bedrock upon which Niels Bohr developed his atomic model. However, while researchers around this time started describing elements in units of nuclear charge, they didn’t really understand why this worked.
It wasn’t until 1920 that researchers actually discovered protons, and until 1932 that the neutron was identified as well. This was the last piece that researchers were missing to truly understand what an atomic number is and what it actually means. Eventually, these insights would lead to the development of nuclear physics, nuclear bombs, and nuclear energy — elements that define the world as we know it today.


