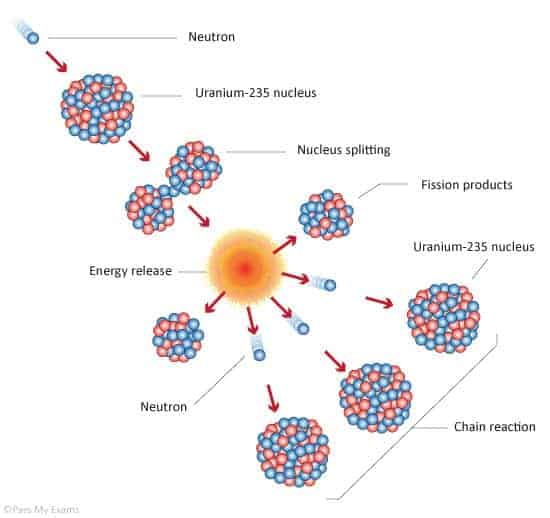
Nuclear reactors transform the energy released by decaying, unstable atoms into electricity. We call such atoms ‘radioactive’. This decaying process is roughly the same one that fuels the Sun, only running in reverse. It can release a huge amount of energy, but it also generates radioactive waste products that are extremely harmful to all life if not handled properly.

Despite their historic hiccups (and the massive damage these caused), nuclear reactors are a modern technological jewel. And yet, they’re not that different from the smart kettle you make tea in at home, just the source of energy is different. With officials and the public increasingly discussing whether (and how) to remake our electricity grids for the future, the topic of nuclear power is bound to pop up as well. So let’s take this opportunity to understand exactly how it works, its strengths, and its pitfalls.
History has shown, again and again, that these reactors are not toys. The processes taking place inside them are driven by fundamental physical forces, and should under no circumstances be taken lightly. Through human and technical error, like with Chernobyl, or just by cruel twists of fate and (maybe) poor foreplanning, like with Fukushima Daiichi, such devices can cause untold death, destruction, and widespread misery if we’re not careful. This, alongside the issue of nuclear waste, is the chief criticism leveled at nuclear energy. But let’s get cracking.
First off, the basics
In the context of our discussion today, ‘nuclear energy’ means fission, the splitting of an atom into two smaller atoms of a different element. Fission is the same reaction that powers our nuclear weapons, the infamous ‘nukes‘.
We’re also hard at work seeking to expand that definition by developing fusion reactors, which generate energy by merging two small atoms into one larger one of a different element. Fusion is what fuels stars and it’s no exaggeration to say that it could give humanity infinite(ish), free(ish) energy. While there is a limit to what fusion can produce, it’s so far above what we’re able to consume today that it’s practically infinite. While it does use fuel, that fuel is hydrogen, the simplest and probably most abundant element in the Universe — so it’s hard to imagine a scenario in which we could really run out of it.
Still, back to our fission. This reaction requires unstable atoms in order to work since we’ll be using that instability to break them apart and extract energy. Radioactive atoms are normal atoms of an element that have too many or too few neutrons at their core. We call them “unstable” because they need to remove these neutrons and/or other subatomic particles in order to revert to ‘stable’ atomic layouts. The different layouts an atom can take are known as its isotopes.
The particles these atoms emit as they decay are the ‘radiation’ that makes them so deadly. There are three kinds of radiation: alpha, beta, and gamma rays, each produced by their corresponding decay process (alpha radiation is emitted during alpha decay, for example). Alpha radiation consists of a helium-4 nucleus (2 protons and 2 neutrons), beta radiation consists of high-energy electrons, and gamma radiation consists of very-high-energy photons. Keep in mind that antimatter should in theory also be able to decay in the same way, generating their corresponding anti-particles (such as positrons instead of electrons).
Radioactive isotopes are naturally occurring, but they’re very rare. The first step needed to use a fusion reactor today is to sift through large quantities of uranium to find these atoms. We do this in devices called centrifuges which separate different isotopes based on their barely-distinguishable weight. The end goal is to concentrate the unstable isotopes in a single place, and the final product is considered to be “enriched”, as in the “enriched uranium” used for fission.
Once you have enough of them put together, you can start making energy (or a bomb). Fission starts with subatomic particles ejected from these radioactive isotopes in an effort to become stable. These are released at a steady rate, which is why every sample of radioactive material, much like sports, has a “half-time” called its half-life. After it is used as fuel, this material is referred to as being “depleted”.
Uranium is the most widely-used fissile element today for several reasons. First of all is that uranium itself is quite abundant, being an estimated 100 times more common than silver. The second reason is that uranium-235 (U-235) is the only naturally-occurring radioisotope present in meaningful quantities in nature that can be split apart relatively easily at room conditions — it doesn’t take a lot of energy or hassle to split U-235 open. Only around 0.7% of all of uranium atoms in nature are of the U-235 variety, and enrichment processes meant for commercial reactors have to increase it to around 3-5% of the atoms in a fuel cell to allow for fission.
What’s a nuclear reaction?
The secret to a good fission reaction is achieving critical mass.
As we discussed earlier, radioisotopes naturally emit radiation, which has a physical carrier in the form of subatomic particles ejected from the atoms. After being kicked out, the particles will keep traveling until they hit something, for example, another radioisotope. This takes place exactly like a teeny tiny car crash would — the two smash together at high speed and bits fly off.
Now, if enough of these collisions take place, the bits flying off will hit other atoms and generate more bits overall. At this point, a runaway fission reaction will start and sustain itself, producing an explosion. To get enough collisions for that you need to have enough unstable atoms in a single place, ready to be hit, meaning a certain weight of the material is needed. That weight is the element’s ‘critical mass’.
Nuclear warheads today act by either compressing their payload to force it to reach critical mass and detonate (pressure and temperature influence a material’s critical mass) or by slamming two bits of fissile fuel together. Kept separate, these do not reach critical mass by themselves, but once they come into contact, you get a very big boom.
This part is also what generates the horrendous, long-term radiation left behind by nuclear weapons. The final microseconds before a nuclear weapon detonates see a massive surge of neutron activation. In essence, huge numbers of neutrons are being released by split atoms and captured by their neighbors nearly instantaneously, which in turn split, generating the nuclear blast. But this process also artificially makes the resulting materials radioactive (they also absorb neutrons but don’t split instantly, and instead decay over time). These materials are then spread by the explosion over vast distances, producing nuclear ‘fallout’.
The only other thing I’d like to point out here is that, essentially, fissile materials hold kinetic energy — motion. The energy we ultimately transform into electricity in a nuclear reactor is that of untold billions of atomic fragments zipping about at immense speed (around 3% of the speed of light for U-235). This motion, ultimately, decays into heat, since heat at the atomic level is just disorderly movement. Materials like coal or gasoline, meanwhile, store chemical energy, which is released as heat through the process of oxidation.
What is a nuclear reactor?
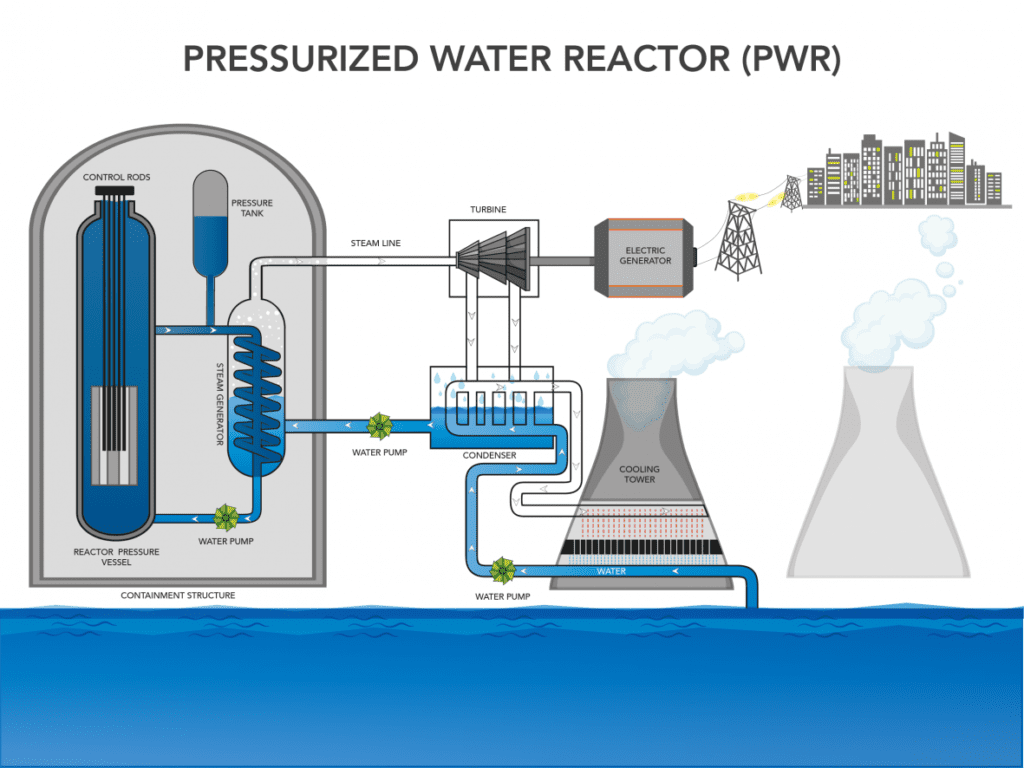
I said earlier that nuclear reactors aren’t very different from a kettle. That’s because such reactors aren’t used to produce electricity per se, they’re used to produce heat, which is converted into steam. It’s this steam that is later used to drive a turbine and generate electricity. It can be used to produce any kind of physical work, such as driving the propellers of aircraft carriers, and can in theory also be used for heating.
Still, that’s where the similarities with kettles end. Nuclear reactors serve a double duty: they need to start the fission process, but they also need to keep it under control. They both create the conditions for fusion to take place and prevent it from running its course. Up to now, I’ve talked of how nuclear weapons behave because they’re simpler devices. But the process that fuels them is the same, the difference is the timescale. Reactors simply don’t let the material release all its energy at once and wipe everything out, but draw this blast out over time to keep it manageable instead.
Exactly how they do this is a matter of design, and we’ve come up with many designs — too many to go through here. For now, just understand that the main job of a nuclear reactor isn’t to start the fission reaction; that’s so simple you could do it in your living room. Their job is to keep it in check.
Even today, our most reliable method of keeping this reaction in check is through control rods. These are bars of “neutron poisons” — materials that absorb neutrons. They are pushed into the mass of nuclear fuel to scrub out neutrons and slow down the rate of fission, and they need to have different properties for different types of nuclear fuel.
Uranium is our go-to fissile fuel. After enrichment, it is pressed into small ceramic pellets which are then stacked together in metal tubes, creating “fuel rods”, which are then bundled together in “fuel assemblies”. Each assembly typically carries around 200 rods, and each reactor will include a few hundred assemblies (although this number will vary based on the desired energy output).
Another critical element of a nuclear reactor is the cooling system. There are several approaches to doing this, but they virtually all use water to drain heat away from the core. The coolant thus serves both to extract energy from the core and as a safety mechanism, as it prevents a meltdown/explosion.

The most common cooling approach in the world today is that of using pressurized normal water (as opposed to ‘heavy‘ water). These reactors, known as pressurized water reactors (PWR), were initially designed for use in submarines. Their operating principle involves pumping water around the core at high pressure, which prevents it from boiling. At the temperatures generated by an operational reactor, this boiling would quickly cause a steam explosion and subsequent meltdown.
While the design can use a single cooling circuit, most commonly they have two. The first feeds pressurized water directly around the core to cool it. The second one has pipes around the first circuit and feeds water at normal pressures. This is meant to transport the heat away as steam to be used.
Having a single cooling circuit is the more energy-efficient and cheaper approach. However, the water that comes in direct contact with the core is contaminated and becomes radioactive. The two-circuit design is thus usually preferred as it limits risks and human exposure to radiation.
What happens when things don’t work out?
Sadly, we have a very good idea of what happens when reactors fail in their primary role, and the fission reaction is allowed to run out of control.
Chernobyl
The Chernobyl nuclear accident is perhaps the most infamous example. As far as we know, one issue of its design was that the plant relied on diesel back-up generators, which took around one minute to start and push control rods fully into the fuel. In the case of a power failure, this would mean that the reactor would go one whole minute without cooling (the pumps were electrical) before the rods could be deployed. This timeframe was considered dangerously long, so they started looking for fixes.
One idea they had was that the residual steam in the plant’s turbines, although lower-than-normal in the case of a failure, could still produce enough power to feed the pumps and keep the reactor stable until the rods could be inserted. The first test was carried out in 1982, after which the system was modified as the turbines couldn’t provide enough voltage for the pumps. Two more tests in 1984 and 1985 also came just short of the desired target window of 45 seconds of power.
In 1986, the year of the meltdown, the test was scheduled during the automated maintenance shutdown of Reactor 4 and focused on developing the best switching sequences needed for the process. Since the shutdown was automatic, and the test was already becoming routine at this point, it was not being directly overseen by the chief designer of the reactor nor the scientific manager, only by the director of the Chernobyl power plant. Meddling with the established steps of the experiment after it had begun led to the historic meltdown. Allthatsinteresting has a more in-depth look at the event here.
Fukushima Daiichi
The Fukushima Daiichi nuclear disaster is less well-known, but it is the latest and much better documented. It is one of the few events of this type to ever be classified as a class 7 on the International Nuclear Event Scale, putting it on par with Chernobyl. This is calculated from the impact on the population, not the severity of a blast or by the quantity of contaminants produced.
Some important wisdom to take from this event is that the tests at Chernobyl were serving a very real and quite sensible goal. The second is that location matters.
Issues at Fukushima started on the 11th of March 2011, when a local earthquake caused the plant’s automatic safety shutdown procedures to kick into place. Because the reactors were shut down, its coolant pumps had to be fed from the secondary diesel generators. So far, so good — troubles are unavoidable, that’s why we have safety systems in place, and we’ve learned from Chernobyl. The real issues, however, were just beginning.

The earthquake also generated a tsunami that was about 14 meters (46 ft) in height by the time it reached Japan’s shore. This went over the station’s seawalls and flooded its lower levels, including machinery serving reactors 1 through 4 and a spent fuel holding area. Worst of all, the emergency diesel engines were located here. They stalled, throttling the flow of coolant. Only reactors 1 through 3 were in operation at this date; reactor 4 had been de-fueled a few months back, while 5 and 6 were shut down for maintenance.
Engineers at the plant attempt emergency measures to keep the reactors from melting down, including freshwater injections into the reactors, venting of steam to the atmosphere to reduce pressure (this is a hail mary as it releases radioactive particles straight from the core into the air), even going so far as to pump seawater laced with boron directly onto the cores. This method is truly a desperate, last-ditch effort, as the boron damages the reactor, making it permanently unusable. Seawater is being carried to and sprayed over the reactor by firefighters and their trucks. Masao Yoshida, the plant’s manager at the time, orders everyone to continue pumping seawater onto the reactors, desperate to keep them from detonating, even after the company which owns the plant, TEPCO, told them to stop.
On the morning of the 12th, reactors 1 and 3 had completely lost their automatic coolant feeds. All their coolant is now provided by truck. Reactor 2 is suffering from lower fluid levels than ideal, but it’s considered to be stable. By evening, reactor 1 suffers a hydrogen explosion, essentially blowing its own lid clean off. The main reactor casing is still operational but there is extensive damage to the building. Five workers are injured. All reactors are being vented into the atmosphere and pumped full of boric acid in a bid to stabilize them by this point. Although unknown at the time, reactor 1 had already completely melted down through its containment chamber and is exposing nuclear material to the atmosphere.
An evacuation order is issued to everyone within a 20 km radius due to high radiation levels at the plant. In the following days, areas around Fukushima would register very high levels of ambient radiation in the air and drinking water.
On March 14, reactor 3 suffers a hydrogen explosion with similar consequences. These two detonations cause extensive damage to the coolant feeds and ventilation systems of reactor 2, severely limiting how much water can be pumped into it. It is now considered the worst-off reactor out of all three. Monitoring and sensor systems around the plant are starting to lose efficiency due to the mounting structural damage and increasingly degraded condition of the reactor cores.
I’ll leave you on this cliffhanger here. There were a lot of things going on at this time — I do strongly recommend you go read about the monumental efforts made at Fukushima, it’s a thrilling story — but it stops being very relevant to ‘reactors’ around this point. Despite these hydrogen explosions and wide-scale radiation contamination of the surrounding area, Fukushima’s core did not explode. Hydrogen explosions are caused by pressure and chemical reactions between super-hot hydrogen in a reactor’s steam and oxygen in the air.
Not a toy, but we’re not playing
Discussions around nuclear fuel invariably pivot to their associated risks, and this is a valid criticism. I’ve listed these two examples here to showcase that we actually do learn from our mistakes. Even if we make new ones, the lessons we’ve had and a bit of luck can help us keep risks contained in the future.
But those risks will never really be zero. The thing that makes nuclear power so dangerous is exactly why it has so much potential — it is incredibly powerful. In every nuclear reactor, engineers are playing a game of cat and mouse with the fundamental forces of reality. What we have to decide, going forward, is whether the risks are worth the payoffs. That being said, there might be a time when only nuclear power can deliver enough energy to feed our ever-growing needs; but we’re not there yet.