A group of scientists at Ames National Laboratory has figured out a way to turn single-use plastic packets, motor oils, and various other petroleum products into environment-friendly, good-quality products. In their latest study, the scientists claim that by using an aluminum reactant and zirconium-oxide-based catalyst, they can change the chemical structure of polyolefin plastics which are basically hydrocarbons comprising long and unbreakable chains of carbon atoms.

According to the researchers, about 50 percent of the total plastic items produced on Earth are polyolefin plastics. These can be found in products ranging from detergents to electronics and even N95 masks. This is why polyolefins are also among the major contributors to our planet’s plastic waste problem. The proposed catalyst offers a cheap and effective way to break down polyolefin plastics.
This zirconium-based catalyst can do wonders
Polyolefins cannot be easily decomposed because these hydrocarbons (called Aliphatic compound compounds) don’t have any additional atoms in their chemical structure to facilitate the addition of extra elements that could trigger their breakdown. So for example, if you bury a plastic bottle today in your backyard, it won’t decay even in the next 100 years because the long chains of carbon that make up the bottle won’t have any additional elements to react with the chemicals released by the soil bacteria. So no change will take place in the chemical bonds holding the bottle together, and therefore the bottle won’t decompose.
However, there is a process called hydrogenolysis using which polyolefins can be upcycled if not fully degraded. This method involves the use of a catalyst to cut through the carbon-carbon bonds and introduce hydrogen atoms. The study authors suggest that until now scientists have been using rare and expensive metals like platinum as a catalyst for this process, making it both an expensive and non-scalable approach.
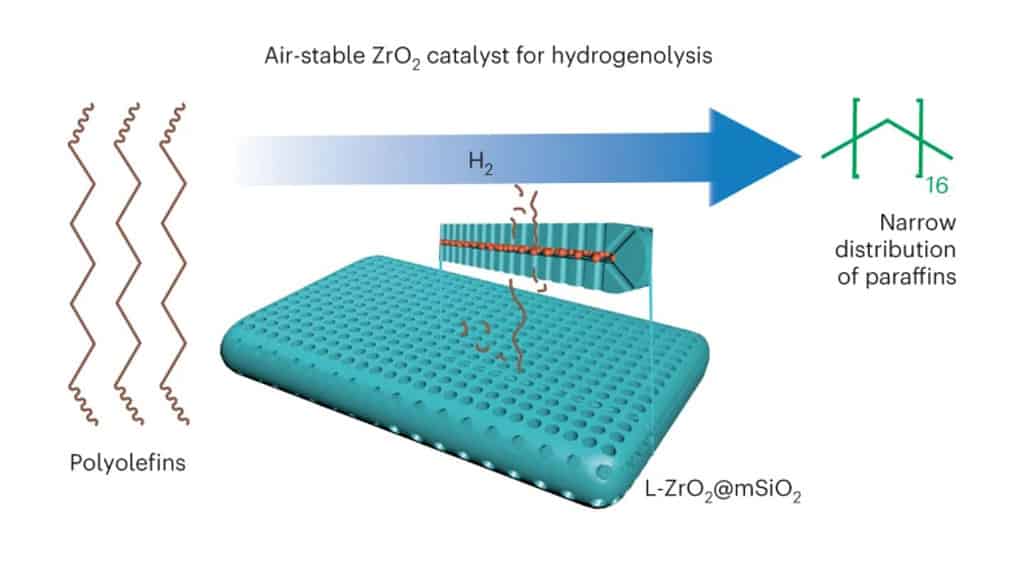
They found that instead of platinum, zirconia, which is a cheap and earth-abundant material, can be considered to break down polyolefins. Since zirconia is less reactive, they designed a sandwich-like catalyst system that incorporated zirconium alkoxide nanoparticles as catalysts placed between two mesoporous silica-alumina plates as reactants. The researchers tested their catalyst system on polyolefin plastics and interestingly, it was able to break down the strong C-C bonds in the material, and do this at speeds similar to that of the platinum catalysts.
“We demonstrate that ultrasmall amorphous localized zirconia nanoparticles (L-ZrO2), covalently embedded in silica and clamped in a void between two mesoporous platelets (L-ZrO2@mSiO2), are highly active in the hydrogenolysis of polyethylene. The architecture (catalyst system) enhances the catalytic activity of zirconia to become comparable to that of Pt/C and improves its selectivity towards liquid products,” the researchers note.
These results excite the authors because they show how to upcycle plastic in a way that is affordable, scalable, and sustainable. They think that by using this technique, they would be able to transform plastic garbage into worthwhile eco-friendly goods. Moreover, the ability to change the structure of hydrocarbons would also allow them to come up with better, stronger, and more durable polyolefin products. With further research, they anticipate being able to employ the catalyst to change polymers in ways that have never before been possible.
Much better than every other method
Zirconia was first used for the purpose of hydrogenolysis way back in the 1990s. At that time, a team of researchers proposed using zirconium hydrides to break down polymers. However, this old approach is not safe because zirconia hydrides are highly sensitive and catch fire upon coming in contact with air and water. So, for example, if you want to disintegrate a plastic bottle using zirconium hydride, you first need to make sure the bottle does not contain even a single drop of water or any moist air. Plus, the environment in which you are performing hydrogenolysis of the bottle should also be free from any impurities.
Aaron Sado who is one of the study authors and currently serving as the director of the Institute for Cooperative Upcycling of Plastics (iCOUP) at Ames, said:
“Harnessing zirconium hydrides for hydrogenolysis is really nice chemistry. The problem is those zirconium organometallic species are really air and water sensitive. So they have to be handled under the cleanest of conditions. Typically polymer waste is not pure and isn’t supplied as a clean and perfectly dry starting material. Using a zirconium hydride catalyst, you’d have to really worry about impurities that inhibit the chemistry.”
Since most discarded plastic items are found in waste, they are filled with impurities and this is why the old approach couldn’t be adopted on a large scale. Other conventional methods that are widely practiced today are either highly energy-consuming or as mentioned earlier, require expensive metals. The current approach, on the other hand, comes with no such limitations. The zirconium-oxide catalyst proposed by the Ames team does not react with water or air, it is not affected by the presence of any impurities, and the catalyst system uses inexpensive and abundant metals.
The researchers will now use their catalyst system to conduct a series of tests involving the addition of different types of functional groups to polymers. All these tests will explore the different ways in which plastic can be upcycled.
The study is published in the journal Nature Catalysis.


