With enough pressure, you can turn anything into metal, and water is no exception. However, scientists Czech Academy of Sciences in Prague managed to turn liquid water into a bronze-like metallic state without having to apply ungodly amounts of pressure, which makes the achievement all the more impressive.

If squeezed together tightly enough, atoms and molecules can become so compacted in their lattice that they begin to share their outer electrons, allowing them to travel and basically conduct electricity as they would in a copper wire. Case in point, in 2020, French scientists turned the simplest gas in the universe, hydrogen, into a metal and fulfilled a prediction made in 1935 by Nobel Prize laureates Eugene Wigner and Hillard Bell Huntington. Metal hydrogen is, in fact, a superconductor, meaning it conducts electricity with zero electrical resistance.
To do so, the French researchers subjected hydrogen to a staggering 425 gigapascals of pressure — more than four million times the pressure on Earth’s surface, and even higher than that in the planet’s inner core. Therefore, it’s impossible to find metallic hydrogen on Earth, although it may very well be found in Jupiter and Saturn, which are mostly composed of hydrogen gas and have stronger internal pressures than the Earth. Likewise, Neptune and Uranus are believed to host water in a metallic state thanks to their huge pressure.
With the same approach, water would require 15 million bars of pressure to turn it into a metal, more than three times the requirement for metallic hydrogen. That’s simply out of our current technology’s reach. However, there may be another way to turn water metallic without having to squeeze it with the pressure of a gas giant’s core, thought Pavel Jungwirth, a physical chemist at the Czech Academy of Sciences in Prague.
Jungwirth and fellow chemist Phil Mason wondered if water could be coxed to behave like a metal if it borrowed electrons from alkali metals, which are highly reactive elements in the 1st group of the periodic table. They got this idea after previously, Jungwirth and colleagues found that under similar conditions, ammonia can turn shiny.
But despite their willingness to go along with this experiment, the researchers faced a predicament. You see, alkali metals are so reactive in the presence of water that they tend to react explosively.
The solution was to design an experimental setup that dramatically slowed down the reaction so that a potentially catastrophic explosion was averted.
Ironically, the key to mitigating the explosive behavior of the water-alki metal reaction was the adsorbtion of water at very low pressure, about 7,000 smaller than that found at sea level. This setup ensured that the diffusion of the electrons from the alkali metal was faster than the reaction between the water and the metals.
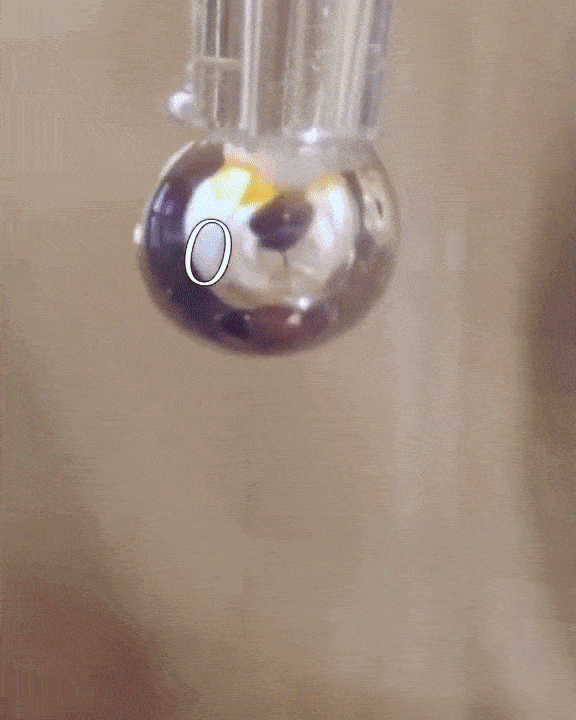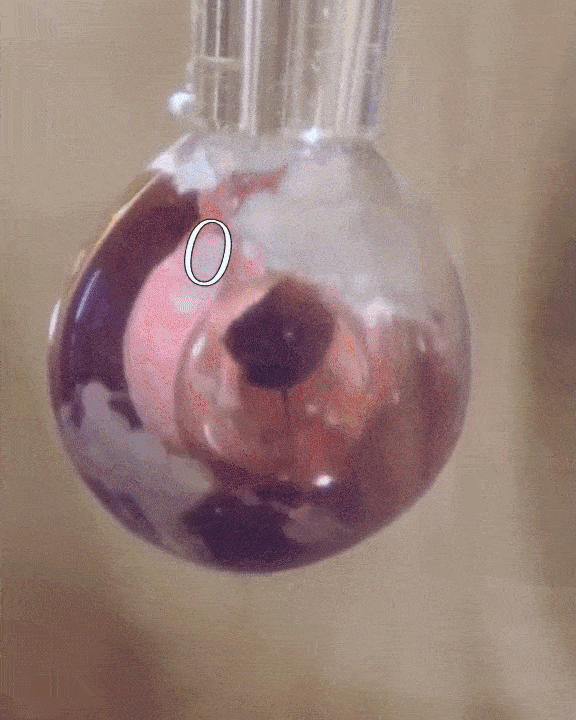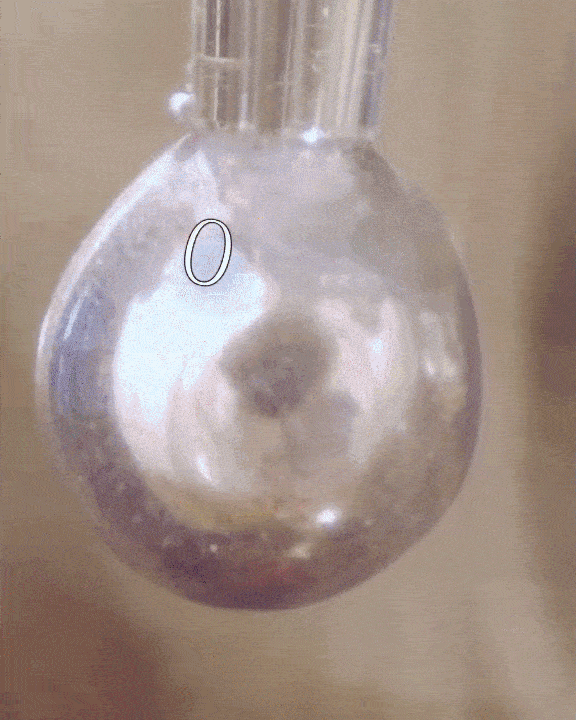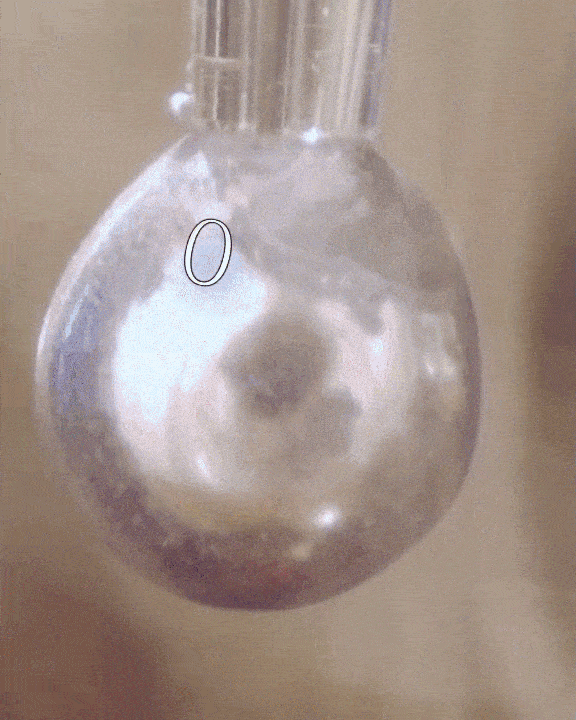
The researchers filled a syringe with an alkali metal solution composed of sodium and potassium, which was placed in a vacuum chamber. The syringe was triggered remotely to expel droplets of the mixture which were exposed to tiny amounts of water vapor.
The water condensed into each droplet of alkali metal, forming a layer over them just one-tenth of a micrometer thick. Electrons from the mixture diffused into the water, along with positive metallic ions, giving the water layer a shiny, bronze-like glow. The entire thing only lasted for a mere couple of seconds, but for all intents of purposes, the scientists had just turned water into metal at room temperature, a fact confirmed by synchrotron experiments.
“We show that a metallic water solution can be prepared by massive doping with electrons upon reacting water with alkali metals. Although analogous metallic solutions of liquid ammonia with high concentrations of solvated electrons have long been known and characterized, the explosive interaction between alkali metals and water has so far only permitted the preparation of aqueous solutions with low, submetallic electron concentrations,” the authors wrote in the journal Nature.









