In 1871, a hot-tempered Russian scientist called Dmitri Mendeleev laid out the most effective grouping of chemical elements. These groupings, which allegedly came to Mendeleev in a dream, organize chemical elements in rows and columns based on atomic number and the orbits of their outermost electrons, respectively.
This early periodic table of elements was not perfect. It predicted eight elements that do not exist, for instance. However, to Mendeleev’s credit, he correctly predicted gallium (used extensively in lasers), germanium, and other increasingly heavy elements. The modern version of this chemical element chart has been tweaked to account for the quantum theory that governs the arrangement of electrons surrounding the nucleus and the outermost electrons that determine an element’s chemical properties.
Filling in The Periodic Table
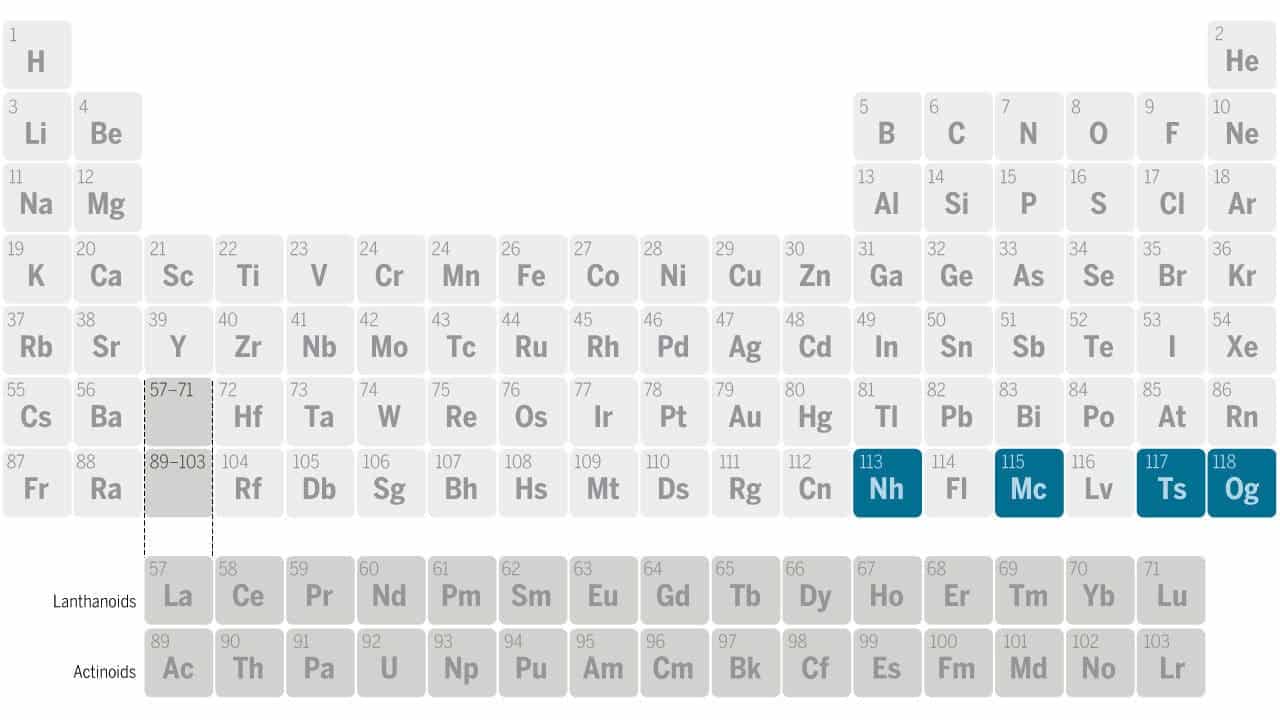
Mendeleev’s table has become as familiar to chemistry students as spreadsheets are to accountants. However, depending on when you were born, the periodic table you learned in school might have had some “blank” spots that have since been filled. Since Mendeleev’s death in 1907, dozens of new chemical elements have been discovered. More recently, in 2017, new elements 113, 115, 117, and 118 were officially instated in the periodic table by the International Union of Pure and Applied Chemistry (IUPAC).

However, don’t hold your breath for the next elements in line like 119 or the even more elusive element 120, also known as Unbinilium. Despite multiple attempts, scientists haven’t been able to synthesize these elements. There are huge challenges involved, including the inherent instability that goes with high atomic numbers and problems with complex experiments involving high-energy particle collisions.
Yet there is still hope. A new study from the Lawrence Berkeley National Laboratory has unveiled a novel, reliable method to produce element 116, livermorium. Previous methods of producing livermorium, which in turn can be used to produce heavier elements like 119 and 120 when bombarded with the right particles, hit a dead end.
A New Era in Element Discovery
After a certain point, elements transition from naturally occurring, such as oxygen and carbon, to laboratory-made. These elements may exist somewhere in the universe, but Earth doesn’t naturally create such conditions. Laboratories like Berkeley’s use advanced technology to pack more protons into atomic nuclei, creating new elements.
The heaviest element to date, 118 oganesson, was created using a beam of calcium isotope 48 particles. However, to make elements 119 or 120, researchers need einsteinium or fermium, which are not synthezied in sufficient quantities.
Enter titanium. Titanium 50, with its stable 22 protons and 28 neutrons, has become the new focus in forging increasingly heavy elements. Researchers reduced titanium oxide to pure titanium and used a special oven to create a beam of ions. Over 22 days, this beam irradiated a plutonium foil, creating livermorium. The stability and performance of the titanium beam exceeded expectations.
“We were very shocked, very surprised, very relieved that we didn’t make any bad choices in setting up the instrumentation,” Jacklyn Gates at Lawrence Berkeley National Laboratory told New Scientist.
Hope Restored
Before this groundbreaking experiment, some chemists had lost hope that they would be able to create element 120. Now, there’s an entire new avenue of chemistry to explore. Creating element 120 may be possible, even if it’s just for a fleeting moment (the stability of atomic nuclei decreases as the mass of an atom increases; positively charged protons repel each other, so the more you pack into a nucleus, the less stable it tends to be).
Lawrence Berkeley National Laboratory scientists plan to start a new experiment aimed at making element 120 in 2025. Many of the steps outlined in this study will be reused, but the plutonium sheet will be replaced with the heavier californium.
One thing is clear: the creation of each new element will become increasingly difficult. The challenge lies not only in detecting these fleeting, short-lived atoms but also in the necessity of using beams of radioactive atoms to forge superheavy elements. While the periodic table may not have a definitive end, our capability to generate new elements might reach its limit.
The researchers presented their findings on 23 July at the Nuclear Structure 2024 conference at Argonne National Laboratory in Illinois.