Life couldn’t exist without some form of energy to power it, and in order to access energy from the environment (i.e. food), animals and plants have had to evolve a conversion process known as metabolism. In a new exciting study, researchers at Rutgers University and Rice University reverse-engineered a primordial protein which might resemble the first biological machines involved in metabolism. In doing so, the researchers have brought us a step closer to uncovering the very origins of life itself.
“We are closer to understanding the inner workings of the ancient cell that was the ancestor of all life on earth – and, therefore, to understanding how life arose in the first place, and the pathways life might have taken on other worlds,” said lead author Andrew Mutter, a postdoctoral associate at Rutgers University’s Department of Marine and Coastal Sciences.
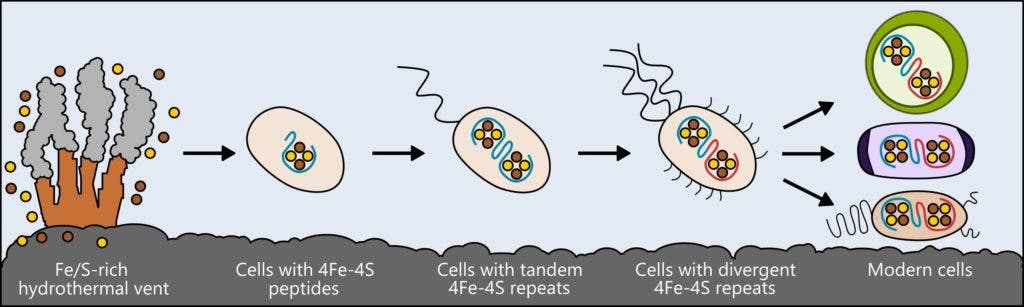
Mutter and colleagues studied a class of proteins called ferredoxins, which play a crucial role in supporting the metabolism of bacteria, plants, and animals by moving electrical charge through cells.
Although today’s ferredoxins are complex, scientists believe that in life’s early days, these proteins had a much simpler form. But what did they look like exactly? Similarly to how biologists compare modern birds and reptiles to infer characteristics about their shared ancestor, the researchers compared various ferredoxins found in all sorts of living things. With the help of computer models, this information enabled the team to design possible forms which the very first metabolic proteins might have taken.
A basic version of the protein was created by the researchers and then inserted into living cells. The researchers first removed the gene responsible for encoding ferredoxin from the E. coli bacteria’s genome, and replaced with a gene for their simple protein. Remarkably, the modified bacteria survived and replicated, although the colony’s growth rate was slower than normal.
The findings have important implications for synthetic biology and bioelectronics, the authors emphasized.
“These proteins channel electricity as part of a cell’s internal circuitry. The ferredoxins that appear in modern life are complex – but we’ve created a stripped-down version that still supports life. Future experiments could build on this simple version for possible industrial applications,” said co-author Vikas Nanda, a professor at Rutgers Robert Wood Johnson Medical School and Center for Advanced Biotechnology and Medicine.
The new study was published in the Proceedings of the National Academy of Sciences.