Researchers from the Weizmann Institute of Science have crafted an “embryo model” that mirrors a 14-day-old human embryo, all without the use of sperm, eggs, or a womb. These sophisticated yet artificial models boast all the hallmarks of this developmental stage, from the placenta to the yolk sac.
Why this is a big deal
The first weeks after a sperm meets an egg are akin to a whirlwind dance of cells, transforming the fledgling embryo from a vague cluster of cells into a form recognizable as a tiny human on an ultrasound. Yet although this pivotal phase is often linked to miscarriages and birth defects, it remains a black box.
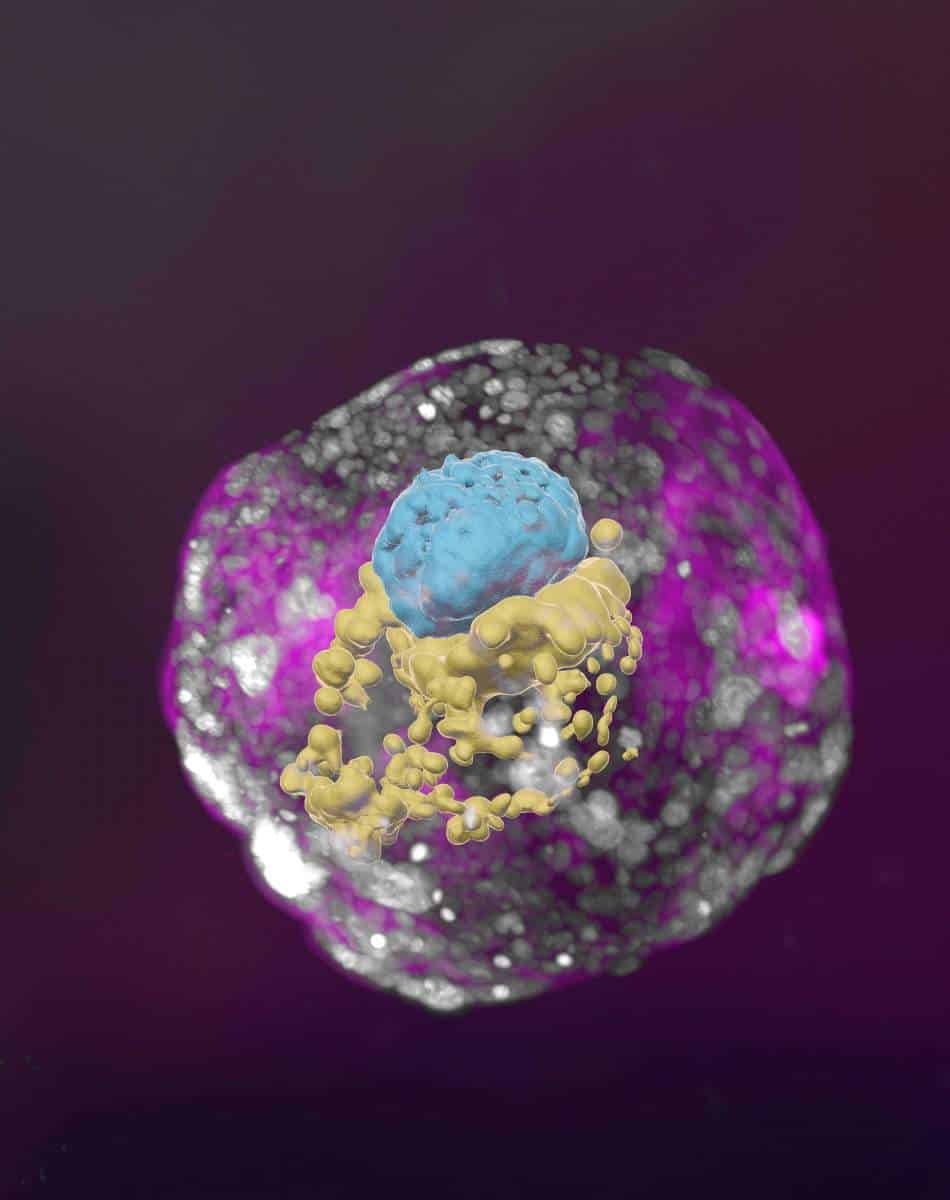
Scientists know the basics of early human embryo development, but the very fine details are still shrouded in mystery. This is why researchers at the Weizmann Institute of Science in Israel have been working on creating models of human embryos in the lab. These cell aggregates — essentially artificial embryos — are capable of organizing themselves into clumps that have all the crucial hallmarks of real viable embryos.
After years of painstaking trial and error, the researchers have now unveiled what is hailed as the first “complete” human embryo model that can replicate the primary structures seen in genuine early embryos produced by egg and sperm.
Previously, cellular aggregates from stem cells fell short of being usable embryo models. They lacked the defining features of a post-implantation embryo, missing key cell types and the structural organization vital for the embryo’s progression. The new model embryo produced by Prof. Jacob Hanna and his team at the Weizmann Institute of Science, however, is a game-changer. It offers a rare window into the embryo’s mysterious early days, which are pivotal for its future growth.
“The drama is in the first month, the remaining eight months of pregnancy are mainly lots of growth,” said Prof. Hanna.
Previously, the Weizmann team grew a mouse embryo outside the womb without the use of sperm or eggs. The artificial embryo grew for a little over 8 days, developing the beginnings of a brain, intestinal tract, and even a beating heart.
Instead of the traditional sperm and egg, the scientists began with naive pluripotent stem cells, some of which came from adult skin cells that were reverted to “stemness.” Picture these cells as blank slates, capable of morphing into any tissue type. With a cocktail of chemicals, these cells were persuaded to transform into four cell types present in the earliest human embryos.
It’s like baking a cake, but instead of flour and sugar, you’re using cells that will become the embryo, placenta, yolk sac, and extraembryonic mesoderm. Mix 120 of these cells in just the right ratio and you get an artificial embryo — and you don’t have to harm any eggs in the process.
“An embryo is self-driven by definition; we don’t need to tell it what to do — we must only unleash its internally encoded potential,” said Hanna.
These embryo models grew until they resembled an embryo 14 days post-fertilization, the legal research limit in many countries. The “architecture” of the artificial embryo has many of the fine details a seasoned fertility expert might recognize under the microscope, from the placenta-like trophoblast to the cavities that would nourish a baby.
Every compartment was perfectly replicated, down to the cells responsible for positive pregnancy test readings. In fact, when the team applied secretions from these cells to a commercial pregnancy test, it tested positive.
Artificial embryos could prove incredibly useful. They could offer scientists valuable clues as to how different cell types differentiate in the crucial first days and weeks of embryo development. About 30% of pregnancies fail in the first week, often without the woman even being aware she was pregnant to begin with.
The bottom line is that human embryos are a lot more challenging to develop than most people think — and the researchers in Israel learned the hard way. Only 1% of their attempts resulted in a working embryo model. Compared to their 99% fail rate, nature has found much more optimal development pathways, which the scientists hope to unravel with time.
Already, the study has unveiled some very good leads, like the importance of placenta-forming cells enveloping the embryo correctly by day 3.
“An embryo is not static. It must have the right cells in the right organization, and it must be able to progress — it’s about being and becoming,” Hanna notes.
With such newfound knowledge, new treatments designed to prevent miscarriages could be on the horizon. There’s also chatter about using these models to enhance the success rate of in vitro fertilization. According to the Society for Reproductive Technology (SART), for women under 35, the percentage of live births via IVF is only 55%.
Ethical implications
The researchers decided to halt the artificial embryo’s development at 14 days. However, they could have legally carried on for more as current rules and regulations only apply to viable biological embryos. These are NOT real embryos.
The scientists stress that their mission is to save lives, not create it. Prior to artificial embryos, the only option for developmental biologists was specimens collected from miscarriages and abortions. More recently, scientists have been allowed to study donated embryos from fertility clinics, but not everyone is keen about this with many countries banning the practice.
These recent developments highlight the need for more robust regulatory framework that can keep up with the pace of innovation in the field.
The new findings appeared in the journal Nature.






