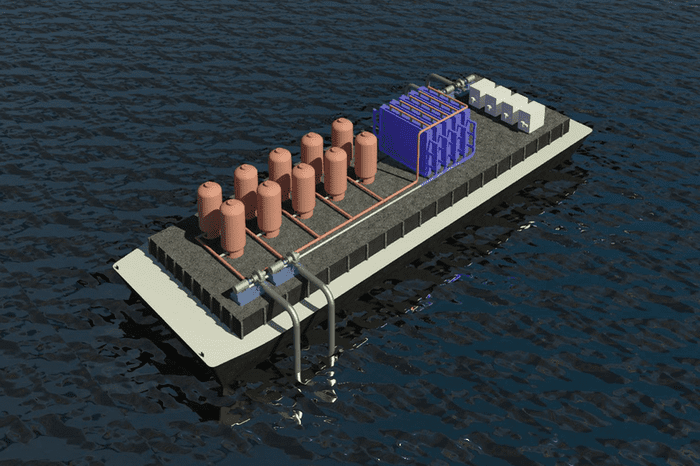
A group of MIT researchers claims to have discovered what could be the most efficient and cost-effective way to remove carbon dioxide from the oceans, the primary “sink” for atmospheric carbon dioxide, absorbing 30–40% of the gas caused by human activity. The results, which were published in the journal Energy and Environmental Science, could be helpful in our fight against climate change.
Carbon from water
In our quest to reduce greenhouse gas emissions, we need all the help we can get.
“The carbon dioxide problem is the defining problem of our life, of our existence,” said Kripa Varanasi, one of the researchers on the project. “So clearly, we need all the help we can get.”
Current technologies for CO2 removal from saltwater use voltage across a stack of membranes to acidify a feed stream via a phenomenon called water splitting. Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen. But in real life, water is not purely hydrogen and oxygen, it includes a number of other substances. The bicarbonates in the water, for instance, are converted to CO2 molecules, which can be removed under a vacuum. The drawback of current methods lies in the complexity and costs as chemicals are needed to drive the total electrode reactions at either end of the stack.
In the new research, the team developed a technique that can be reversed using electrochemical cells without membranes. Through the use of reactive electrodes, protons are introduced to seawater. That is then delivered to the cells, which drives carbon dioxide dissolution. This is a recursive procedure in which acid is added to the water to dissolve inorganic bicarbonates, which are then converted to molecular carbon dioxide and collected as a gas in a vacuum.
The water is then sent through a second set of cells with a negative voltage to extract the protons and neutralize the acidity before it is returned to the ocean. After one set of electrodes becomes proton-depleted (during acidification) and the other set becomes proton-rich (during alkalinization), the roles of the two cells are periodically switched.
Varanasi says that taking out carbon dioxide and putting it back in alkaline water could slowly start to reverse how carbon dioxide is making the oceans more acidic, hurting coral reefs and shellfish. They suggest reinjecting alkaline water via widely spaced outlets or far offshore to prevent an alkalinity rise that could harm local ecosystems.
As with other carbon removal processes, once the carbon dioxide has been taken out of the water, it still needs to be disposed of somewhere. For instance, it may be chemically transformed into substances like ethanol, which can be used as a transportation fuel or into other specialized chemicals. It may be buried in deep geologic formations beneath the ocean floor. The concept would be to integrate such systems with infrastructure that is already in place or is being developed and process seawater (such as desalination plants).
“This system is scalable so that we could integrate it potentially into existing processes that are already processing ocean water or in contact with ocean water,” Varanasi said. “With desalination plants, you’re already pumping all the water, so why not co-locate there? A bunch of capital costs associated with the way you move the water, and the permitting, all that could already be taken care of.”
It would not be necessary to use consumables like chemical additives or membranes there, as carbon dioxide removal could be a straightforward addition to the processes that are already used to return enormous amounts of water to the ocean.
To lessen the sizeable contribution of ship traffic to overall emissions, the system could also be used by ships that would process water as they sailed. International regulations to reduce shipping emissions already exist, and they could assist shipping companies in offsetting some of their emissions and converting their ships into ocean scrubbers.
The system might also be used in places like aquaculture farms or offshore drilling platforms. It might eventually result in the deployment of independent carbon removal facilities worldwide. According to researcher T. Alan Hatton, the procedure might be more effective than air-capture systems because seawater has a carbon dioxide concentration that is more than 100 times higher than that of air. Prior to recovering the gas in direct air-capture systems, the gas must first be captured and concentrated.
“The oceans are large carbon sinks, however, so the capture step has already kind of been done for you,” Hatton said. “There’s no capture step, only release.”
This implies that the amounts of material that must be handled are much smaller, potentially streamlining the entire process and lowering the demands for footprint.
The ongoing investigation has one primary objective: discovering a way to get rid of the carbon dioxide and water that has been separated without using a vacuum. The precipitation of minerals that can foul the electrodes in the alkalinization cell is a problem that affects all reported methods and must be addressed in order to maximize efficiency. Hatton says a lot of work has been put into solving these problems, but it’s too soon to give any results. However, within two years, the team believes the system could be ready for a demonstration project. If that demonstration project is successful, it could be scaled and become deployed in real life.