New research investigated whether early Earth could have supported some of the conditions required for the building blocks of life — as proposed by a famous experiment. Turns out it did.
How did we get here? That’s one of the big questions that has been vexing humans probably since we first became conscious. Thanks to the theory of evolution by natural selection, scientists are confident that our species evolved from a common ancestor that we share with other apes alive today. However, Homo sapiens represents a single twig on a branch of the evolutionary tree that reaches back some seven million years. If you follow these branches all the way to the stem, you’ll eventually reach ground zero: the very first lifeform out of which all other life evolved.
Although the Earth is thought to be 4.5 billion years old, the oldest rocks on the record are about 4 billion years old. Not long after this period, tantalizing evidence of life emerges, including 3.7-billion-year-old stromatolites(layered structures created by bacteria) found in Greenland and 4-billion-year-old stromatolites found in the Labrador Peninsula in Canada.
In 1953, chemists Harold Urey and Stanley Miller conducted one of the most famous experiments of the past century, commonly known as the primordial soup experiment. In order to find out how the first signs of life on Earth surfaced, the scientists exposed a mix of gases to a lightning-like electrical discharge to create amino acids. Amino acids are very important because they form proteins, which, in turn, form cellular structures and control reactions in living things. Remarkably, when water, methane, ammonia, and hydrogen — all chemicals present on early Earth — were hit by the simulated lightning, they reacted to form hydrogen cyanide, formaldehyde, and other intermediate molecules that reacted further to generate amino acids, along with other biomolecules.
But some scientists think that this experiment relies on too many things coming together. Early Earth — whose conditions are still rather poorly understood — was wrapped in a hazy atmosphere which would have made it very difficult for lightning and ultraviolet light to reach the planet’s surface.
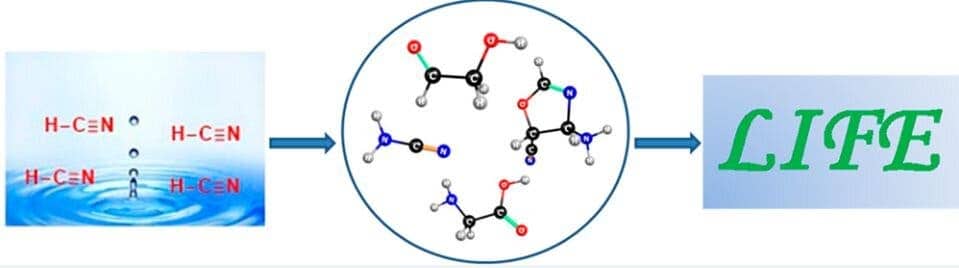
However, that doesn’t mean that there weren’t other alternative forms of energy that could have jump-started these primordial reactions. Indian researchers at the CSIR-National Chemical Laboratory led by Kumar Vanka wondered if heat from ocean waters — which 4 billion years ago were nearly boiling — might have been one such driving force.
In their experiment, Vanka and colleagues used an ab initio nanoreactor that simulates how mixtures of molecules collide and react, forming new molecules. Their results suggest that ancient ocean heat was enough for hydrogen cyanide and water to mix and form the molecules required to produce the amino acid glycine, as well as the precursors of RNA.
Writing in the journal ACS Central Science, the authors conclude that these reactions are both thermodynamically and kinetically feasible, meaning they do not require a catalyst or a lot of energy.
We might never find out exactly how life first emerged on Earth but the fact that there are multiple pathways that could have given rise to it offers some exciting possibilities. It suggests that maybe the conditions necessary for life to form aren’t all that singular, so perhaps many other planets elsewhere in the galaxy and beyond are blessed with this rare gift.


