Researchers at the University of Turku, Finland, report on why the natural mineral hackmanite can change its color when exposed to UV radiation. As this process can occur repeatedly and without wearing the material out, hackmanite could form the basis for new LED and UV-monitoring techniques.
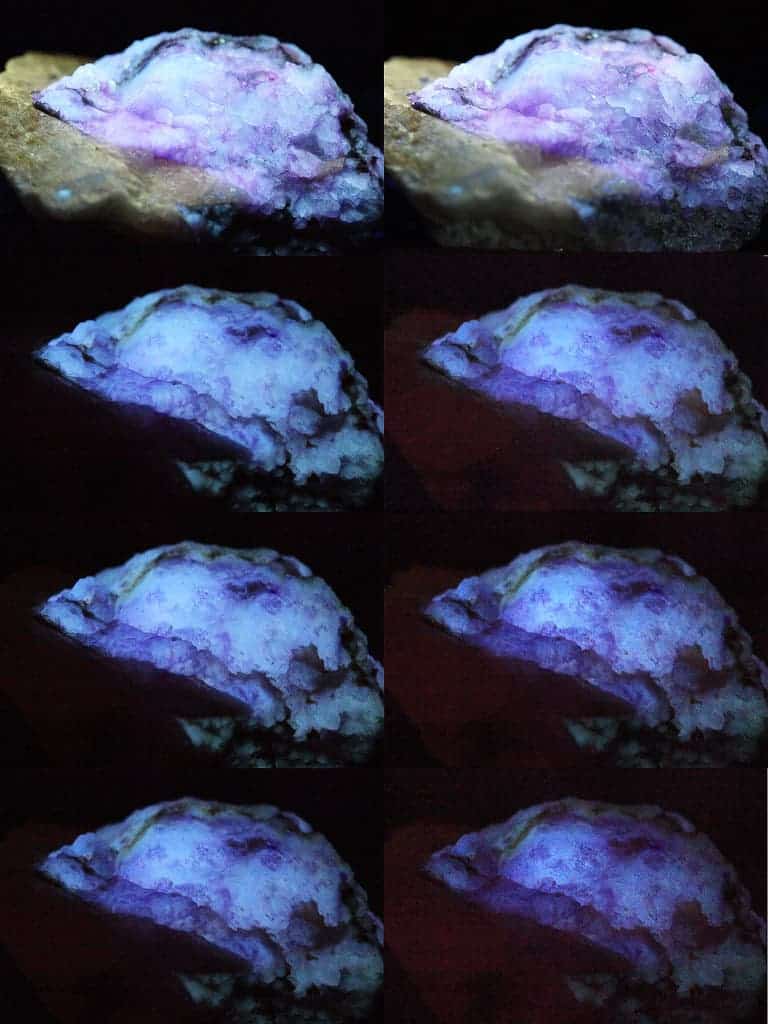
Hackmanite has been under study at the University of Turku for almost a decade now. The mineral is easy to synthesize and has excellent durability. Together with its reaction to UV radiation, these properties make it a very interesting material for researchers looking to leverage its use for applications ranging from consumer electronics to medicinal devices.
What makes it stand out is that hackmanite is one of only three known minerals that can change its color from white to purple when exposed to UV radiation: this process is known as photochromism. Unlike the other two, however, the change in hackmanite is reversible, relatively long-lasting, and doesn’t wear the mineral out in any way.
Exactly why this color change takes place and how, however, has so far remained unknown. The new paper worked with all three natural color-shifting minerals — hackmanite, tugtupite, and scapolite — to find the answer.
Reliably color-change
While the three minerals studied for this paper are all inorganic natural compounds, there are quite a few organic compounds that can reversibly color-change when exposed to UV light as well. These hydrocarbons, however, can only bear through the process a few times before their molecular structure suffers a complete breakdown. This is due to the fact that any perceptible shift in the color of a substance is created by significant changes in its structure, and repeatedly undergoing these changes damages the hydrocarbon molecule.
“In this research, we found out for the first time that there is actually a structural change involved in the colour change process as well. When the colour changes, sodium atoms in the structure move relatively far away from their usual places and then return back. This can be called structural breathing and it does not destroy the structure even if it is repeated a large number of times,” explains Professor Mika Lastusaari from the Department of Chemistry at the University of Turku, Finland, paper co-author.
According to the findings, the three inorganic minerals can seemingly survive this process indefinitely. Their ability to do so stems from their three-dimensional chemical structure. This is similar to that of zeolites, a class of minerals that are used to produce detergents, drying agents, and air purifiers, as their cage-like structure allows them to capture and release different particles. Zeolite detergents, for example, remove magnesium and calcium atoms from water by binding them inside the pores of their cage-like molecules.
“In these colour-changing minerals, all processes associated with the colour change occur inside the pores of the zeolitic cage where the sodium and chlorine atoms reside. That is, the cage-like structure allows atomic movement inside the cage while keeping the cage itself intact. This is why minerals can change colour and revert back to their original colour practically indefinitely,” explains Doctoral Researcher Sami Vuori, paper co-author.
Furthermore, the team explains that the speed with which these minerals can change their color depends on the distance that the sodium atoms inside their structures need to move. This tidbit of information is especially valuable for medical applications, as we now know to more accurately control the color-changing properties of a particular structure.
This is the first time we have a model of how color-changing minerals function, the team explains. The team is now exploring different applications for hackmanite, such as replacing LEDs and other light bulbs or using it for X-ray imaging. Another exciting possibility is the development of hackmanite-based radiation detectors and measuring tools; these would be deployed on the International Space Station and other manned space missions to allow crewmen to measure the radiation uptake of different materials.
“The strength of hackmanite’s colour depends on how much UV radiation it is exposed to, which means that the material can be used, for example, to determine the UV index of Sun’s radiation. The hackmanite that will be tested on the space station will be used in a similar fashion, but this property can also be used in everyday applications. We have for example already developed a mobile phone application for measuring UV radiation that can be used by anyone,” explains Sami Vuori.
The paper “The structural origin of the efficient photochromism in natural minerals” has been published in the journal PNAS.






