The kind of ice we find on the Earth’s surface, from your winter backyard to Antarctica’s giant ice sheets and glaciers, is all the same and has a hexagonal crystal structure. But if you freeze water and compress it at very high-pressure many thousands of times that of Earth’s atmosphere at sea level, the molecular arrangement will be different, depending on how hard you press, resulting in ice with many different crystal structures.
In fact, we know of about 20 different types of ice, each with its unique crystal lattice, and by one estimate there may be more than 300 possible types of ice that await discovery. One such form of ice has now been identified by researchers at the University College London — and it was all thanks to a happy accident during an experiment that may have been more at home at a cocktail bar.
On the rocks

The scientists used a technique called ball milling, in which they vigorously shook ordinary ice with steel balls in a jar cooled to -200 degrees Celsius by liquid nitrogen. The team decided to do this with no specific goal in mind. It was just a cool thing to do, pardon the pun.
But rather than generating small bits of ordinary ice you’d feel comfortable adding to your gin and tonic, this process resulted in a novel type of ice that is unlike all other known ice.
Instead of having a neatly ordered chemical structure, the water molecules are all over the place, meaning it is amorphous. This is the same molecular state occupied by liquid water — but we’re now seeing a lack of molecular organization in a solid. The researchers have called it “medium-density amorphous ice” (MDA).
It’s all pretty mind-boggling, but amorphous solids aren’t unheard of. Glass, plastic, and even gels are all noncrystalline solids in which the atoms and molecules are not organized in a definite lattice pattern. There are also two other known types of amorphous ice, a high-density and a low-density variety, with the newly identified ice occupying the space between them.
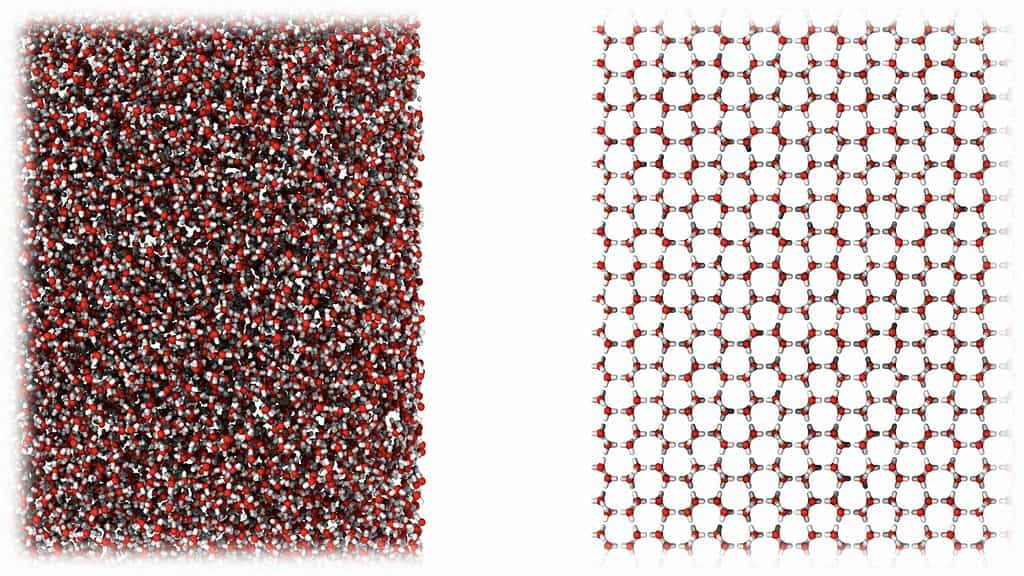
The researchers believe MDA, which looks like a fine white powder, could be found in nature inside the icy moons of Jupiter and Saturn, where the gas giants’ tidal forces exert similar shear forces on ordinary ice as those caused by ball milling. Below the many kilometers of ice on these alien worlds, MDA could trigger tectonic motions and even ‘icequakes’ judging by the extraordinary amount of heat it released when it was warmed up and resolidified.
The researchers have described the newly discovered ice as glassy water, in the sense that it is the precise replica of liquid water in solid form, just like the glass in your windows is the solid form of liquid silicon dioxide.
“We have shown it is possible to create what looks like a stop-motion kind of water. This is an unexpected and quite amazing finding,” said co-author Professor Andrea Sella, a chemist at the University College London.
“Water is the foundation of all life. Our existence depends on it, we launch space missions searching for it, yet from a scientific point of view it is poorly understood,” said senior author Professor Christoph Salzmann of the University College London.
There are many things that are odd and poorly understood about water, notwithstanding the new finding. Water is at its most dense at 4 degrees Celsius but it becomes less dense as it freezes rather than denser as you’d expect. This explains why ice floats on water. Another bizarre behavior is that the more pressure you apply to liquid water, the easier it is to compress, which is totally backward from most other liquids.
During their study, the UCL researchers examined MDA using a range of sophisticated molecular and chemical structure analysis tools, including an electron microscope, X-ray diffraction, and Raman spectroscopy. Next, they would like to subject MDA to high-powered X-rays from a synchrotron to gain more insights into this elusive structure of water.
The findings appeared in the journal Science.






