Fuel cells are absolute wonders of technology – electrochemical systems that directly convert the chemical energy of a fuel (hydrogen and oxygen) into electricity and heat. There’s no combustion, and consequently fuel cells aren’t limited by the same thermodynamic cycles as a typical heat engine. A theoretical efficiency of 70% is thus reached – which is staggering compared to burning fossil fuels. There are numerous hurdles that have prevented so far the hydrogen economy via fuel cells from booming. One such difficulty is the expensive use of platinum as catalysts in the fuel cell.
Researchers at the Max Planck Institute for Solid State Research in Stuttgart report they’ve made a new type of catalyst based on earth-abundant metals (iron and manganese) embedded into organic molecules. The researchers hope the catalyst may be employed as a substitute to platinum, the expensive noble metal.
[ALSO READ] New, affordable fuel cells could spark micro-grid revolution
A new catalyst for fuel cells
Platinum has proven to be essential in driving the key oxygen reduction reaction at the anode side of the fuel cell. Here, oxygen molecules combine cu hydrogen ions and electrons to form water and heat, while an external circuit funnels electrons to an fro the two electrodes, driving an electrical current in the process. Typically, oxygen can combine with either two or four electrons, depending on whether it reacts directly with hydrogen or via an intermediate hydrogen peroxide molecule to form water.
The type of electrochemical conversion we see in fuel cells is far from being an unique man-made wonder. The process can be seen in nature, employed by various biological entities including inside us, humans. As we breath in fresh air, enzymes – which are basically natural catalysts – help drive a combination reaction between oxygen and hydrogen producing energy. The researchers at Max Planck sought inspiration from similar enzymes to replicate in an artificial system of their own.
Such oxygen-reducing enzymes contain metals like iron and manganese, while organic molecules act like sort of anchors for the metals, holding them tight for oxygen to easily bind with them. Klaus Kern and his staff member Doris Grumelli from the Max Planck Institute for Solid State Research vaporized iron and manganese atoms together with organic molecules, in a high vacuum environment, and deposited these on a gold substrate. The resulted molecules auto-assembled and became ordered in patterns that strongly resemble the functional centres of enzymes.
Reducing oxygen – the key
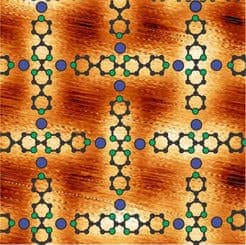
After a bit of toying around for a solution to move the samples into a liquid (transferring samples from high vacuum can be tricky), the researchers eventually landed these on an electrodes surface. It turned out that the catalytic activity depended of the kind of metallic centre, while, on the other hand, the stability of the structure depended on the type of organic molecules that form the network. Iron atoms led to a two-level reaction via the intermediate hydrogen peroxide molecule, while manganese atoms produced a direct reaction of oxygen to water. The reactions took place in an alkaline medium.
Scientists are more interested in a direct reaction, since it’s more efficient, however a hydrogen peroxide reaction could be useful in other applications rather than fuel cells, like biosensors. In any even, the researchers pride themselves with having made a nano-catalyst that is easy to make (vapor deposition is a heavily employed method in the industry) and cheap (readily available metals and organics).
Findings were reported in the journal Nature Communications.


