Helium is the second most abundant element in the universe, second only to hydrogen. Despite its virtual ubiquity, helium isn’t a team player, being totally unreactive with any other element, as any chemistry freshman will tell you. But while helium is by all accounts inert in Earth’s atmosphere, inside the planet’s core is a whole different matter. The extreme pressures cause helium to alter its chemistry allowing it to react with sodium, according to an international consortium of scientists.
The first of the noble elements
Helium is unreactive because it has a closed-shell electron configuration. Since it’s completely full, it’s not happy with sharing or ceding electrons. Such is the case for all noble gases, a select club that also includes neon, argon, krypton, xenon, and radon.
In the early days of experimental chemistry, scientists had thrown everything they had at these elements to spark a reaction but to no avail. In 1924, the Austrian Friedrich Paneth pronounced the consensus. “The unreactivity of the noble gas elements belongs to the surest of all experimental results,” he wrote.
This apparently decisive statement didn’t convince everyone, though, and along the years a number of brave or fool scientists, whichever pleases you, attempted to react noble elements. Finally, in 1962, British chemist Neil Bartlett, working at the University of British Columbia in Vancouver, Canada, succeeded in reacting Xenon with the compound platinum hexafluoride (PtF6), which was made only three years earlier by American chemists. The resulting compound XePtF6 – xenon hexafluoroplatinate — was the first noble-gas compound.
Many other compounds of Xenon and Krypton followed soon after. Then compounds of radon and, in 2000, argon too. In the same year, German researchers made a compound of xenon and gold, the latter being a noble metal which was supposed to be unreactive as well.
Helium, however, given its extremely stable electron configuration has evaded all other previous incursions.
Persistence is key
Taking cues from these earlier inspiring moments in chemistry, an international team of researchers crunched the numbers to see whether or not helium can react with anything. The team was led by Prof. Artem R. Oganov, a professor at Stony Brook University and head of Computational Materials Discovery laboratory at Moscow Institute of Physics and Technology.
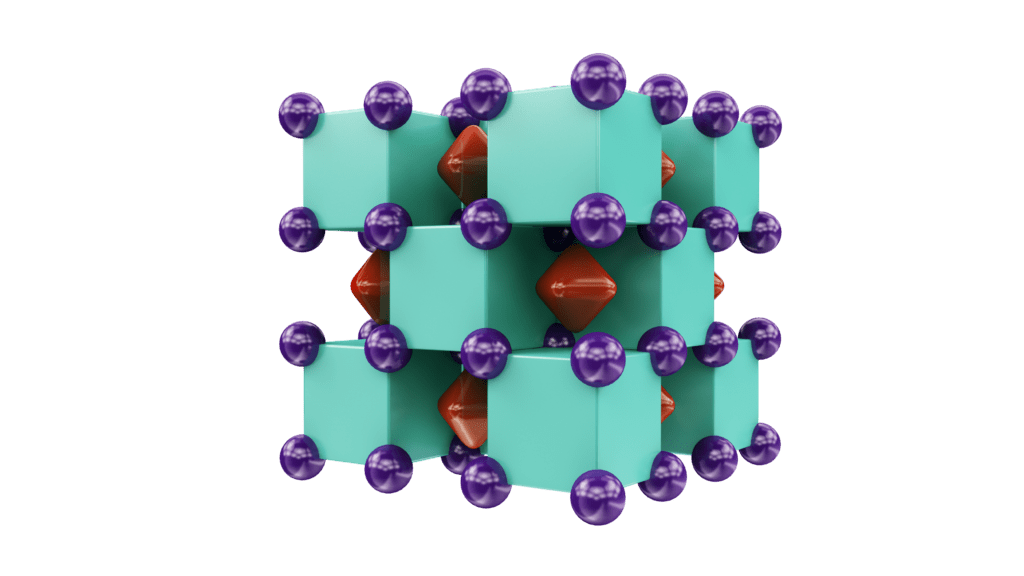
Their computer models suggest that He can indeed form stable compounds, namely Na2He and Na2HeO. Initially, the results suggested that the Na2He compound consists of Na8 cubes, of which half were occupied by helium atoms and half were empty.

“Yet, when we performed chemical bonding analysis of these structures, we found each ’empty’ cube actually contained an eight-center, two-electron bond,” said Utah State University professor Alex Boldyrev. “This bond is what’s responsible for the stability of this enchanting compound.”
“As we explore the structure of this compound, we’re deciphering how this bond occurs and we predicted that, adding oxygen, we could create a similar compound,” said doctoral student Ivan Popov.
After the two He compounds were predicted, the researchers experimentally synthesized them using a diamond anvil cell at the Carnegie Institution for Science in Washington. The compound Na2He formed when the pressure reached 1.1 million times that of Earth’s atmospheric pressure. Predictions suggest the compound can remain stable even at pressures ten million times that. Na2HeO was found to be stable in the pressure range from 0.15 to 1.1 million bars.
“It’s not a real bond” in the sense of the ionic and covalent bonds you learned about in chemistry”, explained Popov. “But [the helium] does stabilize the structure. If you take those helium atoms away, the structure will not be stable.”
A similar technique was used to form metal hydrogen, news which we reported only last week.
Both compounds are ionic crystals — an electride to be precise — with similar structures. Na2He has a positively charged sublattice of sodium ions and a negatively charged sublattice of localized electron pair, which makes the compound an insulator. Na2He has negatively charged oxygen in the form of O2 instead of the electron pairs, as reported in Nature Chemistry.
“The compound that we discovered is very peculiar: helium atoms do not actually form any chemical bonds, yet their presence fundamentally changes chemical interactions between sodium atoms, forces electrons to localize inside cubic voids of the structure and makes this material insulating,” says Xiao Dong, the first author of this work, who was a long-term visiting student in Oganov’s laboratory at the time when this work was done.
This is clearly exotic work. The findings illustrate how the ‘impossible’ is possible sometimes if you set your mind to it.
It was a good day for science!