Brown University researchers reported the development of a copper foam which could turn CO2 into useful chemicals such as formic acid – a preservative and antibacterial agent in livestock feed.
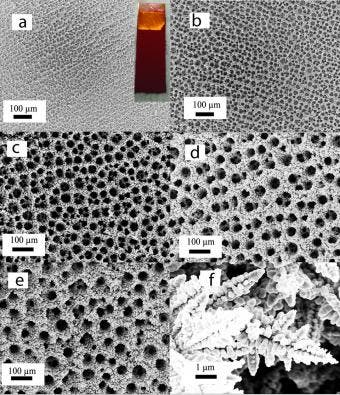
As CO2 emissions continue to grow, scientists are trying to find potential uses to it. The problem with carbon dioxide is that it is extremely stable, so breaking it and making useful industrial chemicals is no easy feat. The catalyst they made from copper foam has “vastly different properties” from catalysts made with smooth copper in reactions involving carbon dioxide:
“Copper has been studied for a long time as an electrocatalyst for CO2 reduction, and it’s the only metal shown to be able to reduce CO2 to useful hydrocarbons,” said Tayhas Palmore, professor of engineering and senior author of the new research. “There was some indication that if you roughen the surface of planar copper, it would create more active sites for reactions with CO2.”
Copper foam was virtually ignored until a few years ago, when it started receiving the attention it deserves. The foam is created by depositing copper on a surface in the presence of hydrogen and a strong electric current. Hydrogen creates bubbles and the copper is deposited in a sponge-like arrangement of varying sizes.
After the foam was created, researchers set out to experiment, and see which chemicals strongly react to it; lo and behold CO2 was one of the winners. Their experiments showed that the copper foam converted CO2 into formic acid much more efficiently than common copper. The reaction also produced small amounts of propylene, a useful hydrocarbon that’s never been reported before in reactions involving copper.
“The product distribution was unique and very different from what had been reported with planar electrodes, which was a surprise,” Palmore said. “We’ve identified another parameter to consider in the electroreduction of CO2. It’s not just the kind of metal that’s responsible for the direction this chemistry goes, but also the architecture of the catalyst.”
To me, it’s remarkable that a material so common and well studied as copper still yields surprises for us. But it’s clear that we still have much to learn about it.
“People have studied electrocatalysis with copper for a couple decades now,” she said. “It’s remarkable that we can still make alterations to it that affect what’s produced.”
Source: Brown University.






