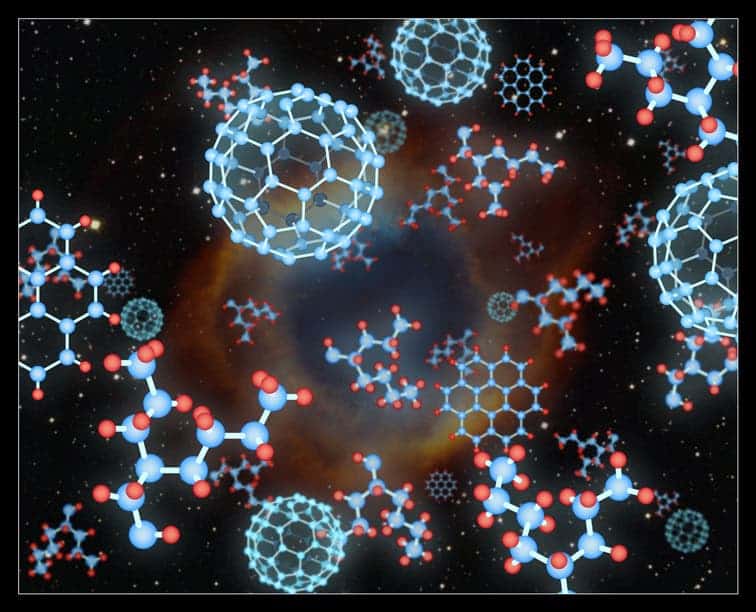
Carbon buckyball molecules rarely exist naturally on Earth. Nonetheless, that did not stop astronomers from finding an unexpected abundance of buckyballs in space. Three years ago, Dr. Olivier Berné and Professor Xander Tielens – then, both at Leiden University – suggested a way to form these carbon buckyballs by sifting the hydrogen from larger carbon-hydrogen molecules.
Now, a team of astrochemists led by Dr. Junfeng Zhen – working in the Sackler Laboratory of Astrophysics at Leiden that is chaired by Professor Harold Linnartz – has experimentally confirmed the underlying part of Dr. Berné and Professor Tielens’s theoretical idea of how buckyballs can form by replicating the process that occurs in space using their own lab equipment.
Fun with Carbon Chemistry
Buckyballs could not exist without carbon’s versatility. Carbon is the most versatile element, allowing carbon molecules to take on many forms, or allotropes.
Molecules consist of multiple atoms chemically bonded together. These atoms can be different elements, as with water (H2O), but they do not have to be, as with oxygen (O2). Though, molecules with only one element are not restricted to just one shape. For example, oxygen can also form ozone (O3), which has a different shape than O2. Oxygen, in its O2 shape, is said to be a different “allotrope” to oxygen as ozone, in its O3 shape. Oxygen also has two other allotropes – O4 and O8 – that are a lot stranger, but far less common.
Meanwhile, carbon has over a dozen different allotropes. Unlike oxygen, these shapes do not just differ by the number of molecules in the allotrope. Instead, carbon allotropes are defined by fundamentally different structures. Here are some examples:
- Diamond – the most loved carbon allotrope – exists in a lattice shape with carbon molecules bonded at tetrahedral angles.
- Graphite – commonly found in pencil lead – exists as sheets or layers of hexagon tessellations stacked and bonded together.
- Graphene – which ZME Science frequently writes about for its many groundbreaking properties – exists as just one single sheet of graphite. Graphene also plays a role as an intermediate step in forming buckyballs in space.
- Fullerenes exist as a variety of “3-D shapes.” Fullerenes with 60 carbon molecules are one form of C60. These 60 molecules can be arranged as 20 hexagons interspersed with 12 pentagons. Can you picture that? This is exactly the same shape as a normal soccer ball, except that fullerenes are hollow and not manufactured by Adidas.
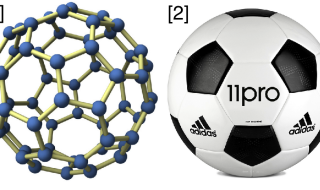
Pathways for Making C60 Buckyballs
To make a molecule with 60 carbon atoms, you need to start with at least 60 carbon atoms. There are two possible ways to get 60 of these atoms. (1) As one option, you can start with a bunch of smaller molecules that each have a few carbon atoms and then combine them together. (2) Or as an alternative, you can start with a larger molecule with at least 60 carbon atoms and then break it apart.
Three years ago, Dr. Olivier Berné and Professor Xander Tielens (Professor Tielens was also involved in this study, while Dr. Berné was not) argued that at least some of the C60 must form from the 2nd method – the breakdown of a larger molecule. The C60 that astronomers have already discovered lives in interstellar space. If the 1st method were the source of the large amount of C60 that astronomers observe, it would require interstellar space to be very dense – dense enough to have a large enough amount of smaller carbon-based molecules to make combining mechanisms likely.
But, interstellar space is defined to be a region with no stars at all. The interstellar space between stars is a vast nothingness composed of just gas and dust. It would need to be ten million times denser to make the 1st method realistic. Thus, the 2nd method – a larger molecule – must be the predominant source for most, if not all of the C60.
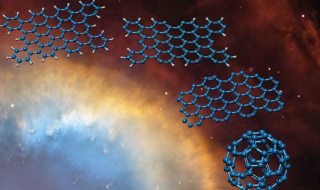
In interstellar space, PAHs are an obvious choice for such a larger molecule. PAHs are hydrocarbons made up of a flat, 2-D hexagon tessellation of carbon atoms with some extra hydrogen atoms attached. Each PAH molecule is essentially graphene with hydrogen atoms attached to the edge of the sheet. Astronomers have long suspected that PAHs are responsible for an unusual band pattern found in most, if not all interstellar spectra. Since the band pattern is so common, this implies that PAHs are nearly everywhere in interstellar space and thus, a good candidate to form C60.
C66H26 is one such PAH that could be broken apart into C60 – presumably with photons. The regions of interstellar space where buckyballs have been found are exposed to large amounts of UV radiation. Some of the radiation hits the PAH molecule, with each UV photon knocking off one of the hydrogen atoms. With 26 UV photon hits, each of the 26 hydrogen atoms is knocked off one by one. At this point, the PAH has become a 66-atom sheet of graphene. The next 3 UV photons will each knock off 2 carbons, leaving exactly 60 carbon atoms left. Then – as the final step – it is natural for the flat graphene that remains to rearrange into a C60 buckyball in this environment.
Breaking Down PAHs Into C60 Buckyballs
In the Sackler Laboratory at Leiden University, Dr. Junfeng Zhen and his team trapped individual C66H26+ PAH molecules using an “ion trap” and subjected them to UV radiation to simulate the conditions in space. They found C60 buckyballs in the products of the experiment, confirming the basis of Dr. Berné’s theory.
The success of the experiments in the Sackler Lab proves two important points. (1) If C60 can form from the breakdown of larger molecules, then other types of molecules in space might also be able to form this way, instead of the usual method of forming through smaller molecules combining together. (2) Forming C60 this way from larger PAHs requires that graphene forms in an intermediate step. Thus, this experiment introduces yet another way to create graphene – this time, by removing the hydrogen atoms from PAH molecules. As with any new method for creating graphene, this may help researchers discover even more of graphene’s exciting properties.
The next time you look up at the night sky into space at the nebulae between stars (and of course, only if you have infrared vision), know that you are also seeing a glimmer from buckyballs in space – and it is not so strange that they are there.
Dr. Olivier Berné’s paper from 2012 on the theoretical study of C60 in space can be found here: http://arxiv.org/pdf/1111.0839v3.pdf
Dr. Junfeng Zhen’s paper on the laboratory experiments confirming Dr. Berné’s work can be found here: http://arxiv.org/pdf/1411.7230v1.pdf





