Radioactive carbon dating determines the age of organic material by analyzing the ratio of different carbon isotopes in a sample. The technique revolutionized archeology when it was first developed in the 1950s, but is currently at risk from fossil fuel emissions.

Image via Pixabay.
Also known as radiocarbon or carbon-14 (scientific notation 14C) dating, the procedure relies on the rarest carbon isotope, carbon-14. Carbon-14 is created on Earth by interactions between nitrogen gas and radiation, usually in the higher levels of the atmosphere. With only 0.0000000001% of the carbon in today’s atmosphere being 14C, it is the rares naturally-occurring carbon isotope on our planet, the others being 12C and 13C.
Unlike the other isotopes, carbon-14 isn’t stable, and it decays over time. Its half-time, the time it takes for half of all 14C atoms in a sample to degrade, is 5,730 years. Putting together that tidbit of information, some very expensive machines, a big of educated guesswork, and ancient tree rings allows researchers to determine the age of a sample of organic material with reasonable accuracy.
Sounds a bit like magic, doesn’t it? Let’s take a look at how it works.
Who thought it up?
The theoretical foundations of radiocarbon dating were laid down by a research team led by American physical chemist Willard Libby in 1949. They were the first to calculate the radioactive decay rate of carbon-14 using carbon black powder. As a test, they took acacia wood samples from the tombs of two Egyptian kings, Zoser and Sneferu, and dated them. Their test showed the wood was cut in 2800BC +/- 250, where earlier independent dating estimated it hailed from 2625BC +/- 75 years — so their method checked out, mostly. There were still some flaws to the approach — the results were slightly fouled by nuclear weapon testing at the time — but these were soon worked out. One of the most important modifications to the initial method was to set the calibration date (we’ll get to this in a moment) to 1950.
For his work, Willard Libby would receive a Nobel Prize for Chemistry in 1960.
How does it work?
Public domain image.
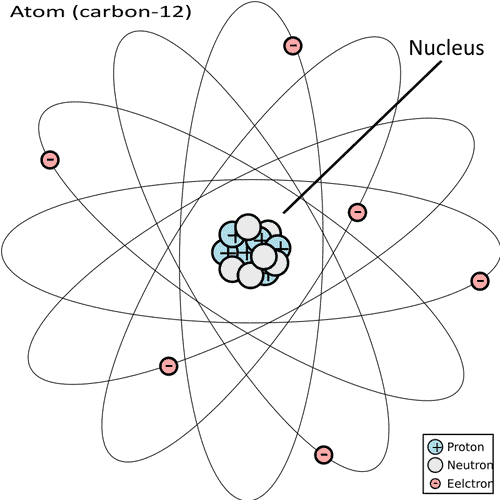
Carbon-14 is continuously created in the upper atmosphere: cosmic radiation ionizes nitrogen-14 gas atoms (which is native nitrogen and has 7 protons and 7 neutrons) into carbon-14 atoms (which have 6 protons and 8 neutrons). Interactions between radiation and the atmosphere at large supply the neutrons that collide with and kick protons out of the nitrogen atom. An atom’s chemical properties (i.e. the element) is a product of the number of protons in its nucleus, not its total mass — more on isotopes here — so this effectively transforms it into carbon-14.
These 14C atoms then get gobbled up by oxygen to become regular CO2, which is consumed by regular plants through photosynthesis, and these plants are then eaten by regular animals and so on. This is the process through which 14C becomes part of all organic matter. 14C has a half-life of 5,730 years, meaning that it takes 5,730 years for half of the 14C atoms in a sample to degrade back into nitrogen-14, and 5,730 more for half of what is left to degrade, and so on.
And here’s the kicker — when these organisms die, they stop taking in new carbon, including carbon-14. Since this latter isotope isn’t stable, it degrades over time. We know the rate it decays it at; so by comparing the current ratio of 12C to 14C atoms in a sample to the initial ratio, we can determine how long ago something died.
How do we do it?
Image via Wikimedia.
There are three main ways to go about it, each with very sciency-sounding names: gas proportional counting, liquid scintillation counting, and accelerator mass spectrometry.
Gas proportional counting measures the amount of beta radiation — the kind of radiation given off during radioactive decay — emitted by a sample. In essence, it involves measuring the level of radiation it emits. Since 14C is the only radioactive isotope in organic material, this effectively tells you how much of it is in the sample. It gets its name from the fact that the sample needs to be transformed into carbon dioxide gas (basically, burned) before this measurement can be performed.
Liquid scintillation counting is another ol’ timer radiocarbon dating technique. It works on the same principle as gas proportional counting but uses different gear. The sample is turned into a liquid form and a scintillator is submerged in it. Scintillators are devices that emit flashes of light upon contact with a beta particle. Two additional devices (photomultipliers) are used to detect these flashes — when both pick up on it, a count is made.
Accelerator (or Accelerated) Mass Spectrometry (AMS) is the modern way of doing things, and over time has become more efficient, faster, and more accurate than the others. It is quite simple, actually — you physically count the number of 14C, 13C, and 12C in the sample using a mass spectrometer. AMS is preferred today for its speed and accuracy, but also because it works with much smaller samples than the other two, helping conserve precious artifacts. For this process, atoms in the sample are ionized (electrically charged) and accelerated using powerful magnets to gradually remove as many atoms not used in the counting as possible. Finally, the carbon-14 isotopes pass into a detector along with some other carbon atoms, and these are used to perform the measurement.
Calibration
“Quite quickly after radiocarbon dating was discovered, it became clear that Libby’s assumption of constant 14C levels in the atmosphere does not hold,” a paper published in 2008 explains. “The level is affected by very many complex factors that have proven impossible to model […] such as: solar cycles, solar storms, geomagnetic variations in the earth, and unpredictable up-welling of old carbon from substantial reservoirs such as oceans. The level has also been impacted by human activity; for example, it increased substantially due to atomic bomb testing in the 1950s and has dropped again more recently due to release of old carbon in fossil fuels.”
“As a consequence, radiocarbon dating is only viable if we can obtain an estimate of the varying level of 14C back through time and can thus plot the function that links radiocarbon ages to calendar ages.”
“Put loosely, we need a calibration curve.”

Image credits Bronk Ramsey / P. J. Reimer et al., (2009), Radiocarbon.
While first developing their method of measuring 14C content, Libby’s team pointed to the possibility that the ratio of 12C to 14C in the atmosphere likely didn’t remain constant over time, but assumed it was as they had no way of correcting for it and wanted to finish their research. As radiocarbon dating saw more use and inconsistencies started to mount, researchers realized that his hunch was right, and set out to ‘calibrate’ the method.
Currently, the calibration date used for radiocarbon dating is the year 1950. In other words, samples are compared against the baseline value of 12C to 14C isotopes recorded in the 1950s. If a sample contains 25% of the carbon-14 you’d expect to see in an organism that died in 1950, it would be two times as old as the isotope’s half-life (so two times 5,730, giving it a rough age of 11,460 years). This isn’t the final age, however.
All the steps we’ve gone through so far don’t actually tell us how old a sample is, just much 14C it contains. As we’ve seen above, accurately dating such a sample hinges on us knowing how much 14C it contained to begin with. To know that, we need to know how much of it was in the atmosphere while the organism lived. This is the process of calibration: changing the assumed initial level of radioactive carbon. It’s perhaps the trickiest bit of the whole process.
“The convention is to assume that the [carbon isotope] ratio has remained constant over time and then to use calibration to compensate for the fact that, in reality, the ratio is changing,” Caitlin Buck, a professor in the Department of Mathematics and Statistics at the University of Sheffield told ZME Science. Professor Buck specializes in applying statistical methods to archeological and paleoenvironmental science and is the co-author of the 2008 paper above.
“At first the need to calibrate seems somewhat unfortunate but, in fact, it allows us to also compensate for several other underlying issues too, like the fact that (at any given time) the ratio in the atmosphere is not the same as that in the oceans.”
The most commonly used and reliable calibration methods are ancient trees. Since trees build a new set of rings every year, they act as 14C archives. From the data in the tree rings, a timeline of 14C levels can be constructed. Timelines from multiple trees would then be compared and overlapped, making this the most accurate record of the isotope we have. The simplest way to go about it would be to find a tree ring that contained the same ratio of radiocarbon as your sample. Other approaches include 14C curves compiled from other sources or to test artifacts that were reliably dated through other means, although this is more of a situational rather than a systemic solution.
Yearly variations in carbon ratios, however, are quite small, which is why radiocarbon dates often come with a “+/- years” variation interval.
Uncalibrated dates are denoted with the unit BP, meaning ‘radiocarbon years before present (1950)’. Calibrated dates use the unit calBP, ‘calibrated before present’. Calibrated dates are the final estimate for the sample’s age, but uncalibrated dates are routinely shown to allow for recalibration as our understanding of 14C levels through time increases. Researchers are putting a huge amount of effort into extending the calibration curve (a timeline of 14C:12C ratios throughout history) and to increase its accuracy. Currently, the curve extends to around 50,000 years ago, but with a relative degree of uncertainty over its oldest reaches.
Limitations and external factors

For starters, if a reliable starting level for carbon-14 can’t be established, radiocarbon dating can’t be used to accurately determine a sample’s age. The technique can only be used to date samples up to around 55-60,000 years old (after which the carbon-14 content drops off to negligible levels). It’s also quite expensive — particularly AMS — due to the very sensitive and highly specialized equipment and personnel needed to run these procedures. Then, there are also external factors that can throw a wrench in the workings of radiocarbon dating.
Carbon-14 is created from the interaction between radiation and the atmosphere, and the advent of nuclear technology (with its plethora of weapon and civilian testing) released a great amount of radiation and radioactive material, driving up the atmospheric ratio significantly.
“The ‘bomb’ samples [i.e. those after 1950] have very high concentrations of 14C, and so if you are working on very old samples for archaeology it is a good idea to have separate extraction lines for the ‘low-level’ samples,” Thure Cerling, a Distinguished Professor of Geology and Geophysics and a Distinguished Professor of Biology at the University of Utah, told ZME Science in an email. “It is quite easy to contaminate ‘old’ samples with ‘modern’ 14C, so a lot of effort has gone into dealing with that issue.”
On the other hand, all that nuclear weapons testing makes it very very easy to date a sample of organic matter that grew during this time, being one of the reasons why 1950 was selected as a calibration date. “Organic material formed during or after this period may be radiocarbon-dated using the abrupt rise and steady fall of the atmospheric 14C concentration known as the bomb-curve,” explains a paper co-authored by Professor Thure in 2013.
He cautions that you must be “very careful” to prevent this kind of contamination, although noting that the issue “is well known” and that “most modern labs have taken sufficient precautions that it is not the problem that it was 30 to 40 years ago.”
Another element affecting this ratio is the use of fossil fuels. Fossil fuels are originated from organic matter, but because they’re formed over millions of years, all the carbon-14 they might have contained has degraded. So when they are burned and their carbon released as CO2 in the atmosphere, it’s pure carbon-12. This further affects the carbon isotope ratio, and does it very fast, impacting the reliability of our dating efforts. Together, these two factors stand testament to the wide reach humanity has achieved over the Earth.
Contamination with external material such as soil can alter the apparent age of a sample by mixing in extra carbon; therefore, all samples are thoroughly cleaned with chemical agents to remove any contaminants. Reservoir effects — this refers to the fact that ocean water contains a different ratio of carbon isotopes than the atmosphere — need to be taken into account when dealing with samples that have been submerged or originate from aquatic environments.
In closing
Radiocarbon dating revolutionized archeology and anthropology by giving researchers a quick and reliable tool to date organic materials. It was a boon to these fields, one whose merits are very hard to overstate. Both Prof Buck and Prof Cerling pointed to the method’s ability to yield absolute age measurements for items of interest — with Prof Cerling saying that it “has revolutionized archaeology” — which allowed us to make heads and tails of historical timelines. Previous approaches such as seriation could only be used to date structures, cultures, and artifacts in relation to one another through the ample application of good, old-fashioned time and labor.
“Likewise it is very useful in determining the age of ice in ice cores that record the history of CO2 and methane in the atmosphere,” Prof Cerling told me.
But radioactive carbon isn’t useful just for dating stuff. Used as a marker molecule, it can allow researchers to, for example, trace specific drugs as they spread through the body, how masses of water move through the oceans, how carbon circulates in nature, and even in forensics to determine when an unknown person died.
Not bad for an unstable isotope of the Earth’s most abundant element.