In transportation, there aren’t that many alternative energy sources like in conventional industry, where you can supply a plant or even a home using solar, hydro or wind power. Before electric vehicles make a significant contribution (don’t hold your breath for too long), alternative means of fueling engines need to be found. This is why biofuels are growing so feverishly, helping cut fossil fuel dependency but at the expense of displacing food crops and, in some unfortunate cases, leading to deforestation. Synthetic fuels have been explored since the turn of the last century, and during WWII Nazi Germany actually used millions of gallons of synthetic gasoline, oil, rubber and such to compensate for loss of oil field control.
Since conventional gasoline and diesel is so cheap, however, attempts at making synthetic fuels at a mass scale have been more or less ineffective. Currently, some 240,000 barrels per day of synthetic fuel are produced, compared to 80,000,000 barrels of crude oil produced every day. Competing with cheap fossil fuel is tough, but by making synthetic fuels cheaper it’s possible to accelerate their share growth.
Making synthetic fuels cheaper and more accessible
Researchers at University of Illinois at Chicago found that using carbon nanofibers doped with nitrogen as a co-catalyst led to a highly efficient conversion of carbon dioxide to carbon monoxide, a useful starting material for synthesizing fuels.
“I believe this can open a new field for the design of inexpensive and efficient catalytic systems for the many researchers already working with these easily manipulated advanced carbon materials,” says Amin Salehi-Khojin, UIC professor of mechanical and industrial engineering and principal investigator on the study.
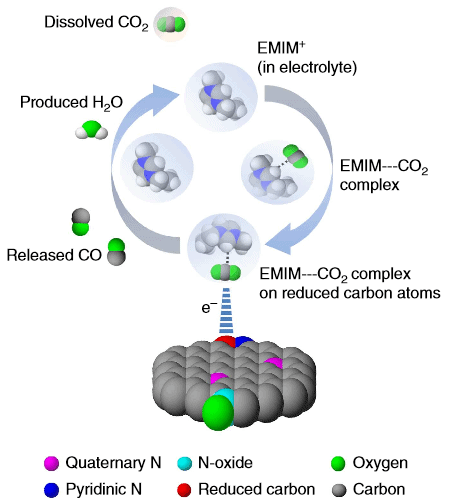
Reducing carbon-dioxide typically involves a two-step process, but despite this chemists have used only one catalyst. Salehi-Khojin and team decided to use a catalyst for each step. Previously, they used an ionic liquid to catalyze the first step of the reaction, and silver for the final reduction to carbon monoxide which rendered a better efficiency than single-catalyst reduction. Silver, of course, is expensive and with this in mind the method doesn’t particularly bring much to the table.
[READ] Synthetic fuels could replace crude oil and cut CO2 emissions by 50%
An unexpected catalyst
The researchers began to explore cheap, metal-free alternatives and finally settled on using carbon nanofiber, a readily available structural material, which was doped with nitrogen in hopes of acting as a silver substitute for the second step of CO2 reduction. Something interesting happened: instead of nitrogen acting a the primary catalysis driver, the carbon nanofibers took charge.
The carbon dioxide reduction ability of carbon nanofibres is attributed to the reduced carbons rather than to electronegative nitrogen atoms. Apparently, thanks to the nanofibrillar structure and high binding energy of key intermediates these nanofibers work as excellent catalysts to the researchers’ surprise and good fortune. Indeed, this is most fortunate since the carbon nanofibers can tweaked to increase catalysis efficiency even further.
“If the reaction happened on the dopant, we would not have much freedom in terms of structure,” said Salehi-Khojin.
But with the reaction happening on the carbon, “we have enormous freedom” to use these very advanced carbon materials to optimize the reaction, he said.
Using their method for the first step reforming process of CO2 to CO, synthetic fuels like syngas may be produced cheaper. Yes, I know what you’re thinking: what about using graphene instead of the graphitic nanofibers?
“Further, one can imagine that using atomically-thin, two-dimensional* graphene nano-sheets — which have extremely high surface area and can easily be designed with dopant atoms like nitrogen — we can develop even far more efficient catalyst systems,” Kumar said.
The findings were reported in a paper published in the journal Nature Communications.






