Ice is water, and water is ice, just in a different form. Intuitively, we tend to think that solid objects are heavier, but that’s not the case at all with ice — and the reason for it is density, along with some really weird water behavior.
Ice floats — that’s why the ocean has polar ice and icebergs, and why the ice in your drink floats. If you think about it, it might seem a bit strange because ice is a solid, and intuitively, you’d expect it be heavier than a liquid and sink. Though this is true for most substances, water is an exception. Its hydrogen bonds and its solid state actually make it lighter than it is as a liquid.
Ice is less dense
Water is an amazing substance that basically fuels life on Earth — every living organism needs it, and we don’t know if life can ever exist without it. But for all its importance, water is pretty bizarre.
Things typically get less dense as they get hotter and denser as they get cooler. Think of it this way: when a substance cools down, its molecules slow down and come closer together. When substances get hotter, they become lighter. That’s why, for instance, when magma inside the planet gets hotter, it rises toward the surface, because it’s lighter and more buoyant; this is basically why we have plate tectonics on Earth. That’s why hot air balloons float (because hotter air is lighter).
But water only behaves like that up to a point.
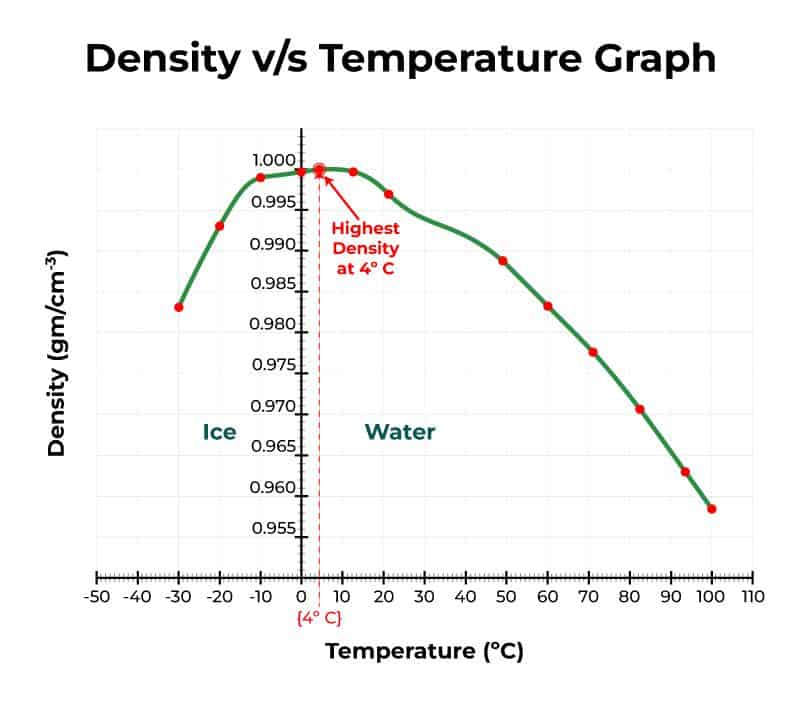
One of the most important properties is that water is the densest at 4 °C (40°F). Water at temperatures both above and below that temperature are less dense. Less dense substances float on top of more dense substances.

Although it seems heavy, an iceberg is less dense than water. Image credits: NOAA’s National Ocean Service.
The reason why ice is less dense than water has to do with hydrogen bonds. As you (should) know, water is made up of one oxygen and two hydrogen atoms. Different molecules are bound by different types of chemical bonds, and atoms in water are bound by something called covalent bonds, that are very strong.
However, another type of bond also forms between different water molecules called a hydrogen bond, which is weaker. These bonds form because the positively-charged hydrogen atoms are attracted to the negatively-charged oxygen atoms of nearby water molecules. When water is warm, the molecules are very active, move around a lot, and form and break bonds with other water molecules quickly. They have the energy to push closer to each other and move quickly.
As water gets below 4 °C, the kinetic energy decreases so the molecules don’t move around so much anymore. They don’t have the energy to move and break and form bonds so easily. Instead, they form more hydrogen bonds with other water molecules to form hexagonal lattice structures. They form these structures to keep the negatively charged oxygen molecules apart. In the middle of the hexagons, there is a lot of empty space.
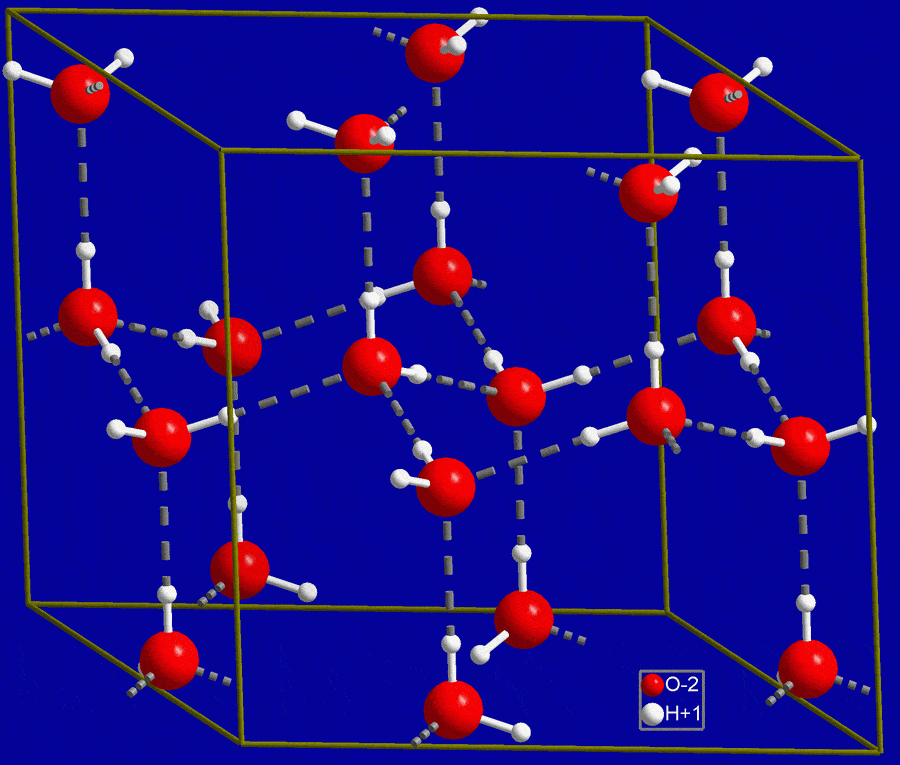
The structure of water molecules as they form ice, notice all the empty space. Image credits: NIMSoffice.
So ice is not as dense as water, that’s why it floats. But it gets even more interesting: the density of water isn’t a straight line, and follows a very peculiar shape. By now, we know that it reaches peak density at 4 degrees Celsius, but it doesn’t decrease linearly or neatly after that.
Ice is actually about 9% less dense than liquid water. Therefore, ice takes up more space than water. Practically, this makes sense, because ice expands. It’s why you shouldn’t freeze a glass bottle of water and why frozen water can create bigger cracks in concrete. If you have a liter bottle of ice and a liter bottle of water, then the ice water bottle would be lighter. The molecules are further away from each other at this point than when the water is warmer. Therefore, ice is less dense than water and floats.
When ice melts, the stable crystal structure collapses and is suddenly denser. As water warms past 4 °C, it gains energy and the molecules move faster and further apart. So hot water also takes up more space than colder water and it floats on top of the cooler water because it is less dense. It’s like when you go to a lake to go swimming and the top layer is nice and warm but when you stick your legs below it is suddenly much colder.
Important for our Earth
So there you have it: ice floats because it is less dense than water, and it is less dense because of the way its atoms are bound and how the molecules arrange themselves at different temperatures. It’s a fascinating example of how the properties of matter can change based on its physical state, and how these changes are not always intuitive.
So why does this even matter? Well aside from being a funky peculiarity of water, ice’s buoyancy has important consequences for life on earth.
Lakes freeze over on the top in the winter in cold places, which allows fish and other animals to survive below. If the bottom froze, the whole lake could be frozen and almost nothing could survive the winter in the lake. In the northern or southern oceans, if ice sank, the ice caps would all be at the bottom of the ocean, preventing anything from living there. The ocean floor would be full of ice. Additionally, polar ice is important because it reflects light and keeps our planet from getting too warm, and ice acts as a sort of blanket, insulating the water and keeping temperatures more stable in harsh winters.
Ice’s lower density also plays a role in shaping the Earth’s surface. Glaciers and ice caps are able to move and flow over the landscape in part because their weight is supported by the underlying ice, which is floating on water. As glaciers move, they erode rock and carve out valleys and fjords, creating glacial landscapes that support a variety of plants and animals.