Every time you bathe in the sea, you have geology to thank for the extra buoyancy that salty water provides. Large-scale geological processes bring salt into the oceans and then recycle it deep into the planet. The short answer to ‘why is the ocean salty’ sounds something like this:
Salts eroded from rocks and soil are carried by rivers into the oceans, where salt accumulates. Another source of salts comes from hydrothermal vents, deep down on the surface of the ocean floor. We say “salts” — because the oceans carry several types of salts, not just what we call table salt.
But the longer answer (that follows below) is so much more interesting.

In the beginning there was saltiness
As it is so often the case in geology, our story begins with rocks and dirt, and we have to go back in time — a lot. Billions of years ago, during a period called the Archean, our planet was a very different environment than it is today. The atmosphere was different, the landscape was different, but as far as ocean saltiness goes, there may have been more similarities than differences.
Geologists look at ancient rocks that preserved ancient water (and therefore, its ancient salinity); one such study found that Earth’s Archean oceans may have been ~1.2 times saltier than they are today.
At first glance, this sounds pretty weird. Since salt in the seas and oceans is brought in by river runoff and erosion, the salts hadn’t yet had time to accumulate in Earth’s earliest days. So what’s going on?
It is believed that while the very first primeval oceans were less salty than they are today, our oceans have had a significant salinity for billions of years. Although rivers hadn’t had sufficient time to dissolve salts and carry them to oceans, this salinity was driven by the oceanic melting of briny rocks called evaporites, and potentially volcanic activity. It is in this water that the first life forms on Earth emerged and started evolving.
“The ions that were put there long ago have managed to stick around,” says Galen McKinley, a UW-Madison professor of atmospheric and oceanic sciences. “There is geologic evidence that the saltiness of the water has been the way that it is for at least a billion years.”
The ancient salinity of oceans is still an area of active research with many unknowns. But while we don’t fully understand what’s going on with the ancient oceans, we have a much better understanding of what drives salinity today.
So how do the oceans get salty today?
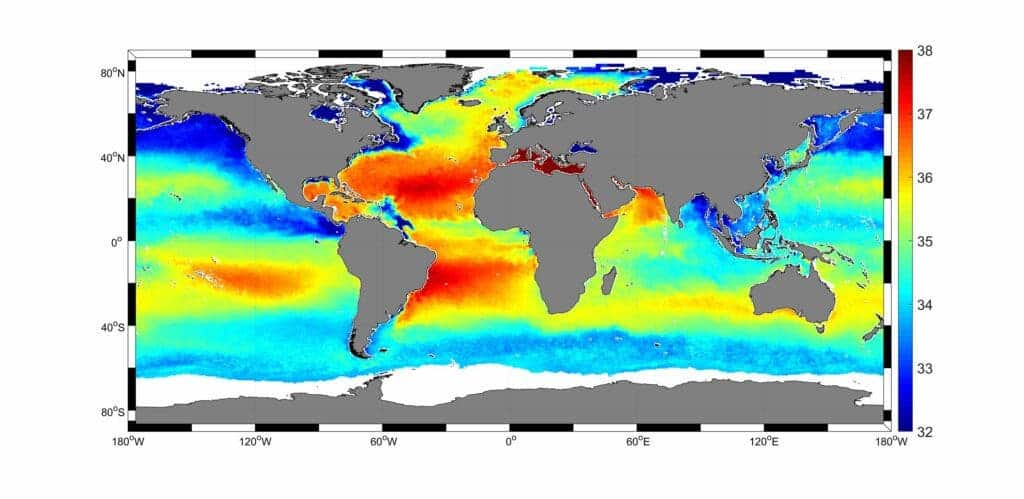
Oceans today have an average of 3.5% salinity. In other words, 3.5% of the ocean’s weight is made of dissolved salts. Most, but not all of that is sodium chloride (what we call ‘salt‘ in day to day life). Around 10% of the salt ions come from different minerals.
At first glance, 3.5% may not seem that much, but we forget that around 70% of our planet is covered in oceans. If we took all the salt in the ocean and spread it evenly over the land surface, it would form a layer over 500 feet (166 meters) thick — a whopping 40-story building’s height of salt covering the entire planet’s landmass. That’s how much 3.5% means in this particular case.
All these salts come from rocks. Rocks are laden with ionic elements such as sodium, chlorine, and potassium. Much of this material was spewed as magma by massive volcanic eruptions and can form salts under the right conditions.
Because it is slightly acidic, rainwater can slowly dissolve, erode rocks. As it does so, it gathers ions that make up salts and transfers them to streams and rivers. We consider rivers to be “freshwater”, but that’s not technically true: all rivers have some salt dissolved in them, but because they flow, they don’t really accumulate it. Rivers are agents for carrying salts, but they don’t store salts themselves.

Rivers constantly gather more salts, but they constantly push it downstream. Influx from precipitation also ensures that the salt concentration doesn’t increase over time.
Meanwhile, the oceans have no outlet, and while they also have currents and are still dynamic, they have nowhere to send the salts to, so they just accumulate more and more salt. Which leads us to an interesting question.
So, are the oceans getting saltier?

No, not really. Although it’s hard to say whether oceans will get saltier in geologic time (ie millions of years), ocean salinity remains generally constant, despite the constant influx of salt.
“Ions aren’t being removed or supplied in an appreciable amount,” says McKinley. “The removal and sources that do exist are so small and the reservoir is so large that those ions just stay in the water.” For example, she says, “Each year, runoff from the land adds only 0.00005 percent of total ocean salts.”
A part of the minerals is used by animals and plants in the water and another part of salts becomes sediment on the ocean floor and is not dissolved. However, the main reason why oceans aren’t getting saltier is once more geological.
The surface of our planet is in a constant state of movement — we call this plate tectonics. Essentially, the Earth’s crust is split into rigid plates that move around at a speed of a few centimeters per year. Some are buried through the process of subduction, taking with them the minerals and salts into the mantle, where they are recycled. The movement of tectonic plates constantly recirculates material from and into the mantle.
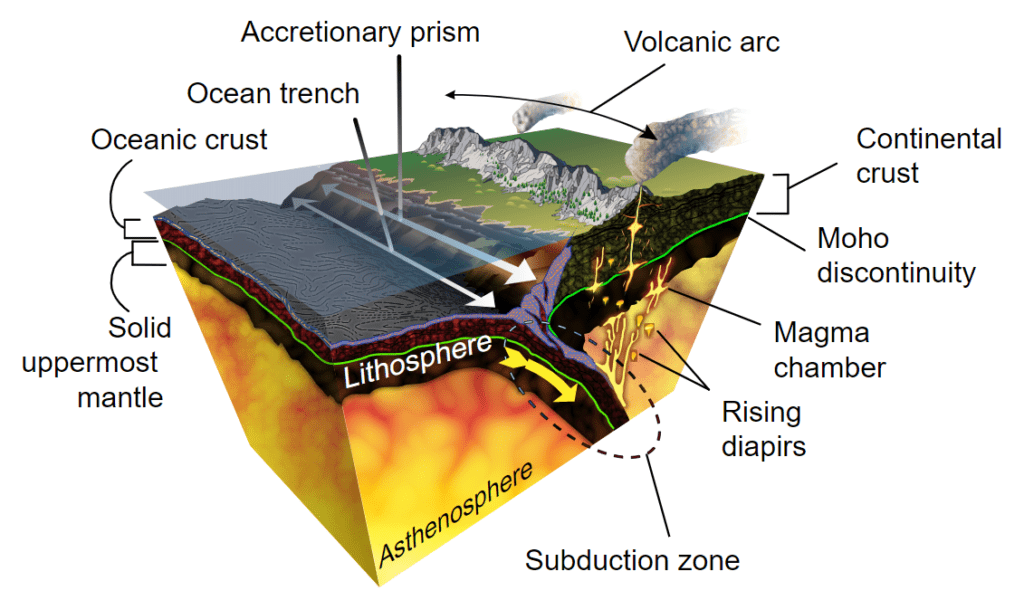
With these processes, along with the flow of freshwater, precipitation, and a number of other processes, the salinity of the Earth’s oceans remains relatively stable — the oceans have a stable input and output of salts.
But isolated bodies of water, however, can become extra salty.
Why some lakes are freshwater, and some are *very* salty
Lakes are temporary storage areas for water, and most lakes tend to be freshwater. Rivers and streams bring water to lakes just like they do to oceans, so then why don’t lakes get salty?
Well, lakes are usually only wide depressions in a river channel — there is a water input and a water output, water flows in and it flows out. This is called an open lake, and open lakes are essentially a buffer for rivers, where water accumulates, but it still flows in and out, without salts accumulating. Many lakes are also the result of chaotic drainage patterns left over from the last Ice Age, which makes them very recent in geologic time and salts have not had the time to accumulate.

But when a lake has no water output and it has had enough time to accumulate salts, it can become very salty. This is called a closed lake, and closed lakes (and seas) can be very salty, much more so than the planetary oceans. They accumulate salts and lose water through evaporation, which increases the concentration of salts. Closed lakes are pretty much always saline.
We mentioned that world oceans are 3.5% salt on average. The Mediterranean Sea has a salinity of 3.8%. The Red Sea has some areas with salinity over 4%, and Mono Lake in California can have a salinity of 8.8%. But even that isn’t close to the saltiest lakes on Earth. Great Salt Lake in Utah has a whopping salinity of 31.7%, and the pink lake Retba in Senegal, where people have mined salt for centuries, has a salinity that reaches 40% in some points. The saltiest lake we know of is called Gaet’ale Pond — a small, hot pond with a salinity of 43% — a testament to just how saline these isolated bodies of water can get.

It’s important to note that lakes are not stable geologically, and many tend to not last in geologic time. Some of the world’s biggest lakes are drying up, both as a natural process and due to rising temperatures, drought, and agricultural irrigation.
Salt can also come from below

We’ve mentioned that rock weathering and dissolving makes oceans salty, but there is another process: hydrothermal vents.
A part of the ocean water seeps deeper into the crust, becomes hotter, dissolves some minerals, and then flows back into the ocean through these vents. The hot water brings large amounts of minerals and salts. It’s not a one-way process — some of the salts react with the rocks and are removed from seawater, but this process also contributes to salinization.
Lastly, underwater volcanic eruptions can also bring salts from the deeper parts to the surface, affecting the salt content of oceans.


