Researchers have developed a new technique that could provide long-term defense, possibly even a cure, for HIV patients. The method calls for HIV-antibodies to be anchored to immune cells, creating a population of resistant cells which can then take the fight to the virus.
Image credits C. Goldsmith / CDC.
Scientists at the Scripps Research Institute (TSRI) may have found a way to hit HIV where it hurts it the most — by taking away the virus’ ability to infect white blood cells. The work is remarkable for its shift in the way antibodies are deployed against HIV. Instead of launching a full-body (but low density) flood of antibodies which float freely in the bloodstream, TSRI researchers led by study senior author Richard Lerner, M.D., Lita Annenberg Hazen Professor of Immunochemistry at the institute, have developed a way in which antibodies can be piggybacked directly on white cells. These active compounds will be tied to the same receptors HIV uses to enter the cells, making out immune system finally immune from the dreaded virus.
“This protection would be long term,” said Jia Xie, senior staff scientist at TSRI and first author of the study.
It comes down to something Xie calls the “neighbor effect.” As the old adage goes, one antibody in hand is worth a hundred five capillaries away. Ok, I may have taken some liberty with that but the underlying idea is that concentrating antibodies to first and foremost defend white cells allows our bodies to join in on the fight — regular treatments can’t do that, and they’re left with a lonely uphill battle against HIV. Even worse, in case HIV is flushed out of the system but the patient gets infected again, his or her immune system will be just as abysmal at fighting off the virus.
Safety first, advances second
Before tailoring the technique against HIV, the team worked with rhinovirus (the bug responsible for most common colds) as a test subject. A lentivirus was used as a vector to deliver a set of genes to a culture of human cells, instructing them to manufacture antibodies and bind these to the receptor rhinovirus ties to (ICAM-1). The rhinovirus was then unleashed upon the culture, but with no access point inside the cells it shouldn’t infect most of them, the theory goes.
That ‘most’ is exactly why they didn’t start testing off the bat with HIV. Gene delivery systems are basically dumbed down viruses with edited genomes, which have to infect cells and paste their genetic data inside the host’s genome. But no system can reach all cells, so the culture became a mix of edited (immune) and non-edited (vulnerable) cells. The point of the experiment was to see how the colony as a whole would fare.
It actually went pretty good. There was an initial shock of about two days when the majority of cells died off. Control cultures, which held only unedited cells, never recovered after the infection. Mixed cultures, however, got back to about the same numbers as prior to infection after about 125 hours — only this time, they were all descendants of the most resistant cells. In essence, the team forced the cultures to go through an evolutionary crash course in the lab dish. All the vulnerable cells were consumed in the infection and the resistant cells multiplied and passed off their anti-rhinovirus genes along.
“This is really a form of cellular vaccination,” said Lerner.
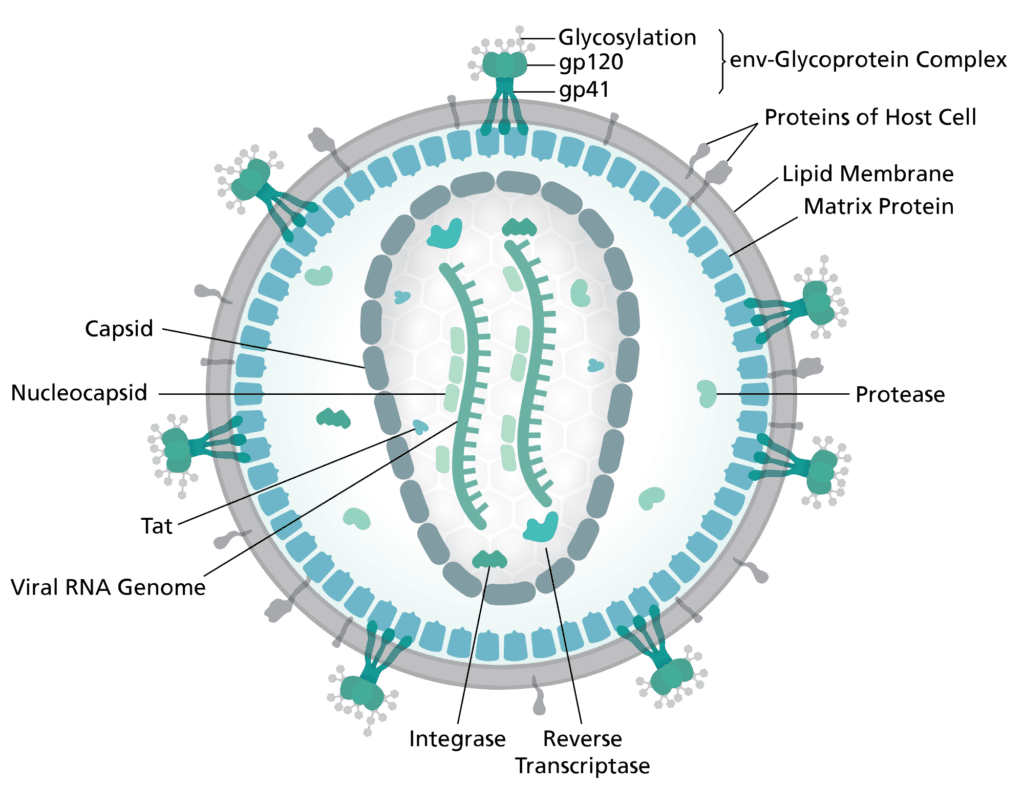
Image credits Thomas Splettstoesser.
Next, they used the same system against HIV. The team tested a number of antibodies to find one which could protect the CD4 receptor (the one HIV uses) on immune cells, copied the corresponding genes in the lentivirus, then let them loose upon the culture. And again, it worked. They further showed that these tethered antibodies worked more efficiently at blocking HIV than free-floating antibodies in another experiment led by study co-authors Devin Sok of the International AIDS Vaccine Initiative (IAVI) and TSRI Professor Dennis Burton.
TSRI now plans to collaborate with researchers at City of Hope’s Center for Gene Therapy to get early efficiency and safety testing done prior to human trials, as per federal regulations.
“We at TSRI are honored to be able to collaborate with physicians and scientists at City of Hope, whose expertise in transplantation in HIV patients should hopefully allow this therapy to be used in people,” Lerner added.
The paper “Immunochemical engineering of cell surfaces to generate virus resistance” has been published in the journal Proceedings of the National Academy of Sciences.


