It’s the first trial in a series of vaccines against COVID-19. It’s still early, but it’s one of the most impressive efforts in medical history.
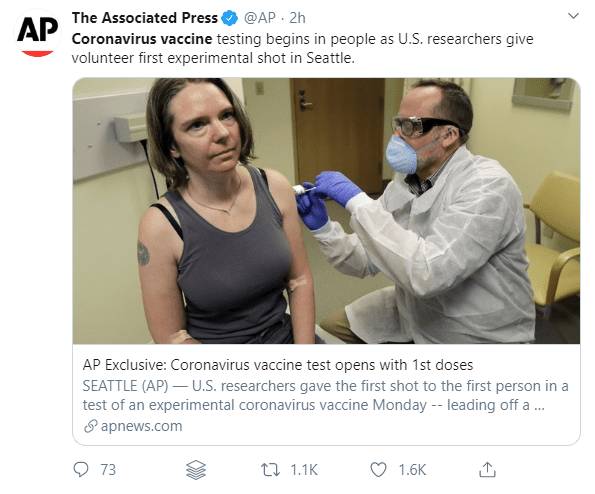
US researchers have administered the first dose of a vaccine candidate called mRNA-1273. Developed by the National Institutes of Health and Massachusetts-based biotechnology company Moderna Inc, mRNA-1273 has officially begun human trials.
The anxiously-awaited study kicked off at the Kaiser Permanente Washington Research Institute in Seattle. The trial will give 45 volunteers two doses, a month apart, in order to test the vaccine’s effectiveness in protecting subjects against COVID-19. If it is successful, further studies will be carried out to look for common side effects or other possible complications.
“We’re team coronavirus now,” Kaiser Permanente study leader Dr. Lisa Jackson said on the eve of the experiment. “Everyone wants to do what they can in this emergency.”
The trial kicked off in record speed. It’s a milestone achievement, showcasing just how much effort and resources have been poured into developing treatments for COVID-19.
This isn’t even the only such effort. Dozens of research groups around the world are racing to develop treatments or vaccines for the coronavirus. It’s a momentous effort, as it is absolutely necessary to gain a foothold against the virus. Going from not even knowing there was a virus to having a vaccine in a couple of months is unprecedented in the entire medical history, Jackson told AP.
However, it’s still early days, Jackson emphasizes.
“We don’t know whether this vaccine will induce an immune response, or whether it will be safe. That’s why we’re doing a trial,” Jackson stressed. “It’s not at the stage where it would be possible or prudent to give it to the general population.”
The trial will feature carefully chosen volunteers; some will receive lower doses, some will receive higher doses, to test how strong the inoculation should be. Previous tests on mice are encouraging, but going from mice to humans is always a big leap, and often not a successful one.
But it’s important to think in perspective. Even if everything goes right, and this trial is successful, even if everything is fast-tracked, we are still at least 12 months away from mass production, and even farther away from a realistic vaccine-produced immunity.
However, since we don’t know how the disease will evolve and there is evidence that it will last for at least a few months, a vaccine couldn’t come soon enough.
Editor’s recommendations:









