Researchers at the Weizmann Institute of Science in Israel have made a huge leap in synthetic biology after they grew a mouse embryo outside the womb without the use of sperm or eggs. The tiny embryo was entirely made from stem cells, raising interesting but also ethically challenging possibilities that might one day lead to breeding a live animal — and that may include humans — solely from cultured stem cells in a lab. For now, though, this is a fantastic demonstration of biotechnology that will help scientists gain a better grasp of how stem cells work and how they might help us cure various diseases.
Life begins when egg meets sperm — or so we thought
How a tiny blob of cells transforms itself into a complex biological structure complete with many specialized tissues and organs is one of the most baffling mysteries of biology. In the last couple of decades, biologists have been taking apart embryos virtually cell by cell to unravel each stage of their development in the most intricate detail. Over the years, scientists have learned a lot about the kinds of cellular signals and physical forces that shape embryos and their supporting cast of tissues.
Out of all studied mammals, the mouse molecular instructions manual has been researched the most, with scientists disabling genes one at a time to test what each of them does. In time, the instructions were clear enough that scientists grew confident they could grow an embryo from scratch.
In 2019, a research team at the University of Texas Southwestern Medical Center was the first to use extended pluripotent stem cells to develop an embryo without any sperm or egg, which they then implanted and grew inside a female mouse. Only 7% of the implants were successful but those embryos that did work actually started developing early fetal structures, though there were some major malformations.
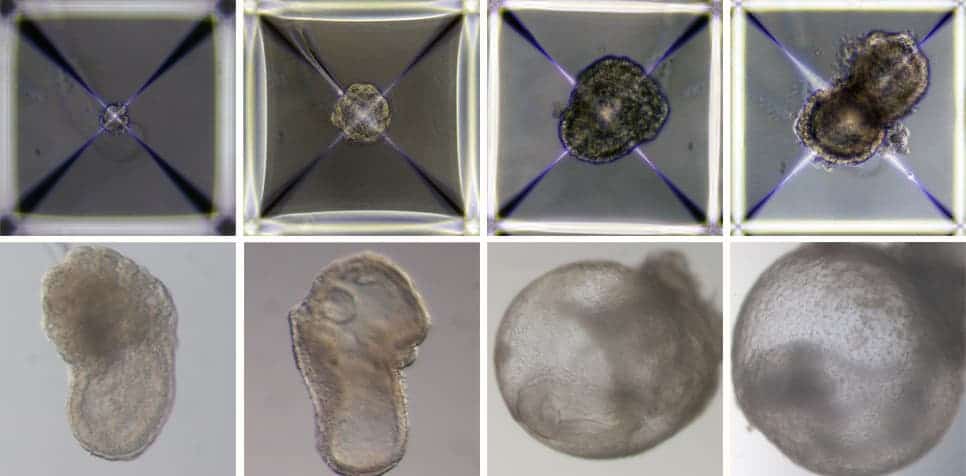
Now, researchers at the Weizmann Institute went a step further, developing artificial embryos outside the womb, all entirely in the lab. First, they reprogrammed stem cells into a so-called “naive” state that allows them to develop into specialized cells. Some of these stem cells were cultured to develop into embryonic organs, while others were genetically modified to turn into cells for the placenta and yolk sac.
All of these cells were clumped together inside a complex mechanical uterus that mimics the natural incubator of a mouse womb, and which the scientists spent more than seven years developing. The artificial womb carefully controls pressure, oxygen exchange, and nutrient flow to simulate the environment required for a mouse embryo to develop.
The synthetic embryo grew for a little over 8 days, developing the beginnings of a brain, intestinal tract, and even a beating heart. The gene expression patterns of the synthetic embryo matched natural ones to within 95%. Previous efforts that attempted to grow embryos outside the womb inside test tubes and dishes couldn’t sustain development for more than a few days at most.
However, the vast majority of stem cell aggregates never made it to this highly advanced stage. Only 50 out of about 10,000 synthetic embryos, or 0.5%, reached eight days of full development, which is nearly half of the mouse gestation period.
The very high failure rate shows just how complicated this whole process can be and how much there is still to learn. Nevertheless, the new research represents a tremendous achievement, which could pave the way for making viable embryos from scratch, and potentially a living organisms too. Armed with such knowledge, scientists could grow working human organs for transplantation entirely in the lab, starting from matured cells donated by the patients themselves, thereby ensuring 100% biocompatibility. Animal testing would also be greatly reduced or even rendered obsolete.
“The embryo is the best organ-making machine and the best 3D bioprinter – we tried to emulate what it does,” said Professor Jacob Hanna, lead researcher on the study. “Instead of developing a different protocol for growing each cell type – for example, those of the kidney or liver – we may one day be able to create a synthetic embryo-like model and then isolate the cells we need. We won’t need to dictate to the emerging organs how they must develop. The embryo itself does this best.”
The findings appeared in the journal Cell.



