By now, many people have heard Russia’s announcement: the “first coronavirus vaccine to receive regulatory approval.” The announcement seemingly came out of nowhere, since there were no previous announcements of large-scale trials.
Unfortunately, the announcement did come out of nowhere. It’s easy to approve vaccines, but ensuring that they work and they are safe is a completely different beast. Russia has done the former, but not the latter.
Like drivin a car without testing the brakes
If you’ve followed other commentary about the vaccine announcement, you’ve probably seen a lot of negativity, and I’m afraid this one won’t be any different.
In June, the Gamaleya Research Institute of Epidemiology and Microbiology (an institute working under the Health Ministry of the Russian Federation) registered a combined Phase 1 and 2 trial for a vaccine on 38 volunteers. Just two months later, it’s been given regulatory approval.
“Regulatory approval” sounds more trustworthy than it actually is. It’s not an international standard or some sort of widely-accepted approval — it only means that the vaccine has been approved in Russia; and to approve the vaccine after only a couple of months of human testing, without any large scale trials, is not acceptable in modern medicine. There’s just no responsible way to approve this.
Now, the vaccine itself is not more advanced than other candidates and thankfully, we have quite a few candidates. The first vaccine clinical trials started in March and there are 29 candidates at the moment, including vaccines from Moderna, Novavax, Pfizer, AstraZeneca, and of course, the one from the Gamaleya Research Institute, which has now received regulatory approval.
Some of these are already reporting encouraging early results and more candidates are expected to launch soon. We’re seeing unprecedented progress in vaccine development and we’ve already pulled out a lot of the regular stops, specifically to accelerate the development of a vaccine. Some researchers are already concerned about the safety regulations for the upcoming vaccines, even as candidates from AstraZeneca or Moderna only seem to cause mild side effects.
But here’s the thing: without large-scale trials, there’s just no way to tell if the vaccine is safe or how well it works.
Why Phase 3 trials are so crucial
Despite recent antivaxx propaganda, vaccines are some of the safest and most effective medical procedures out there. They’ve saved over 20 million lives in the past 20 decades alone, and the number is growing every year. Undoubtedly, a coronavirus vaccine would also save many lives.
But you can’t skip safety steps. There’s a reason why right from the get-go, researchers said that around one year is the best we can hope for when it comes to developing a vaccine — and even that’s optimistic.
Vaccine studies start well before any human is injected. They’re tested on cells, then on mice and primates, and only if everything works out fine, human trials commence. Many don’t make it this far.
First, the vaccine is given to a few volunteers in a controlled setting, where researchers monitor the participants.
This is the Phase 1 stage. Its main objective is to see if the vaccine is safe to assess for efficacy — it doesn’t say anything about how well it works, just that it can be tested on a larger sample size. If Phase 1 goes alright, then the vaccine is given to a few hundred volunteers. This is Phase 2.
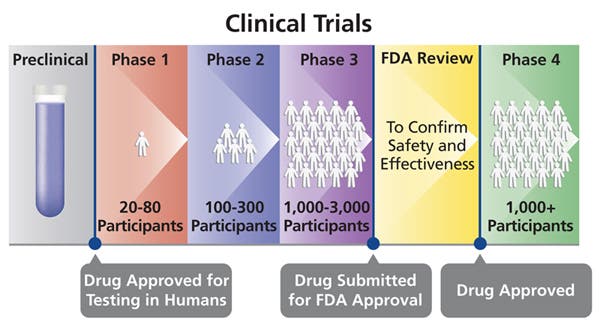
When Phase 2 starts, researchers try to make the first assessments of the experimental vaccine. They make detailed observations about immunity, toxicity, and any side effects, but it’s still a relatively short-term observation. It’s only in Phase 3 that a full assessment is carried out.
In Phase 3, thousands or tens of thousands of volunteers receive the vaccine, and results are compared with a placebo in a large, randomized control trial. Researchers then wait for them to encounter the virus in the real world and note any results and side effects, determining vaccine efficacy and safety. Just because a drug or vaccine has reached Phase III doesn’t mean that it’s guaranteed to pass it.
Around half of all drug candidates fail during Phase III or are rejected by regulatory agencies due to their side effects. Overall, the success of vaccines is less than half for industry candidates, and 7% for non-industry candidates.
By all accounts, the Russian vaccine could have, at most, completed Phase 1 and 2 trials. This is the stage that several other promising candidates are at, but that’s not reason enough to start using them yet.
Experimental treatments? In some cases. Experimental vaccines? Never
It’s worth asking, since several experimental treatments have already been tested, why we can’t do the same thing with vaccines. After all, if we fast-forwarded things like Remdesivir, why couldn’t we do the same with vaccines?
The fundamental difference is that these treatments are given to people who are already sick, and usually very sick. Vaccines are meant to be administered to millions and millions of healthy people. Even if the vaccine works, and even if it has only rare side effects, when you multiply “rare” by millions you can end up doing more harm than good. Even after vaccines pass Phase 3, they are constantly monitored because it’s possible that some groups of people were not included in the trials, and they could develop adverse effects.
These tight measures are what makes vaccines so safe and reliable, and they’ve been completely bypassed in this case.
Can the vaccine actually work? Sure, it’s a fairly promising candidate. But it’s far too early to put it to work, and even in the case of the ongoing pandemic, the risks far outweigh the advantages.
Scientists around the world immediately denounced the certification as premature and inappropriate, and the Russian response at the criticism has been a bizarre mixture of nationalism and criticism for international experts. For instance, one article from state-owned Sputnik claimed:
“However, the news was not taken as positively as one might have expected in the Western media, which doubted the drug’s safety and effectiveness – and even its existence, citing the accounts of various “experts”. Many of them pointed to the lack of medical data from human trials available to the public, as well as the small size of the test groups, which amounted to 76 people, not counting the members of the Gamaleya Research Institute who worked on the vaccine and, confident in its effectiveness, voluntarily injected themselves with it.”
In addition to ironic quotes around “experts”, the last part (underlined for clarity) is particularly concerning. If all the employees did indeed “voluntarily” inject themselves with it as they were monitoring the symptoms, that’s unscientific at best, and unethical on multiple levels. Even if we entertain this idea, as far as we could find, the institute still has fewer than 400 employees, not enough for a clinical study, and no results from this alleged trial have been presented.
In an article on the vaccine’s new website, Russian officials try to justify the vaccine using Russia’s vaccine leadership “for centuries”, mentioning that Russian Empress Catherine the Great received a smallpox vaccination in 1768, “before the United States”. It’s not clear what this comparison is meant to accomplish, especially since it goes more than 250 years in the past, and the US was only founded in 1776.
If the announcement is meant to put Russia’s achievements in a good light, it’s doing the exact opposite.
This is not the time to peddle unproven solutions and hollow claims. As Derek Lowe notes, this announcement takes us backward rather than forward and only works sow confusion and discord, which is the last thing we need — unless creating confusion and discord is exactly the intent.



