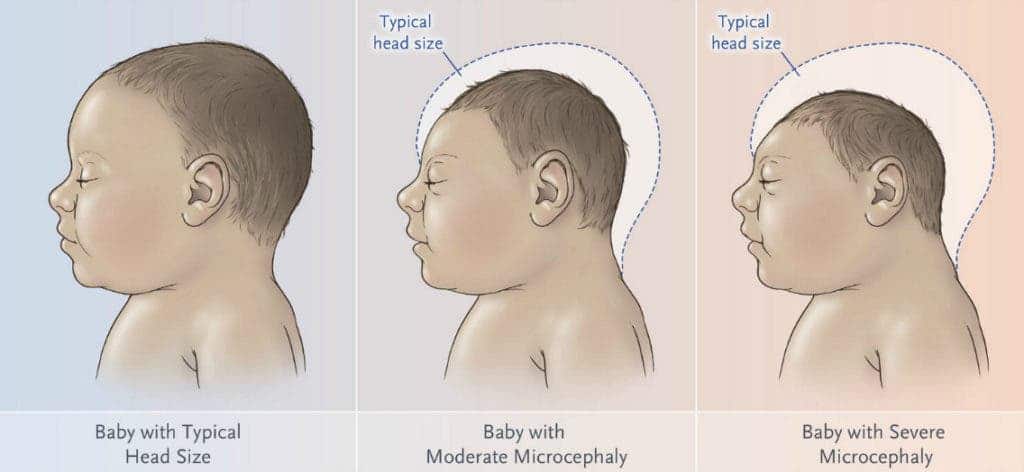
In the early part of 2016, the World Health Organization’s Emergency Committee (EC) under the International Health Regulations (2005) (IHR 2005) discussed the clusters of microcephaly and Guillain-Barré Syndrome (GBS) cases that have been temporally associated with Zika virus transmission.
Brazil, France, the United States of America, and El Salvador provided information on a potential association between microcephaly and other neurological disorders with Zika virus. The recent cluster of microcephaly cases was considered a Public Health Emergency of International Concern (PHEIC). Several months later, the WHO confirmed in a scientific consensus that the Zika virus is linked with microcephaly as well as Guillain-Barré syndrome.
Three years and several studies later, researchers at Baylor College of Medicine revealed one way how in utero Zika virus infection can lead to microcephaly in newborns. The team discovered that the Zika virus protein NS4A interrupts the growth of the brain by taking control of a pathway that regulates the generation of new neurons.
Rare genetic mutations helped explain how Zika causes microcephaly
“The current study was initiated when a patient presented with a small brain size at birth and severe abnormalities in brain structures at the Baylor Hopkins Center for Mendelian Genomics (CMG),” said Dr. Hugo Bellen, professor at Baylor, investigator at the Howard Hughes Medical Institute and Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital.
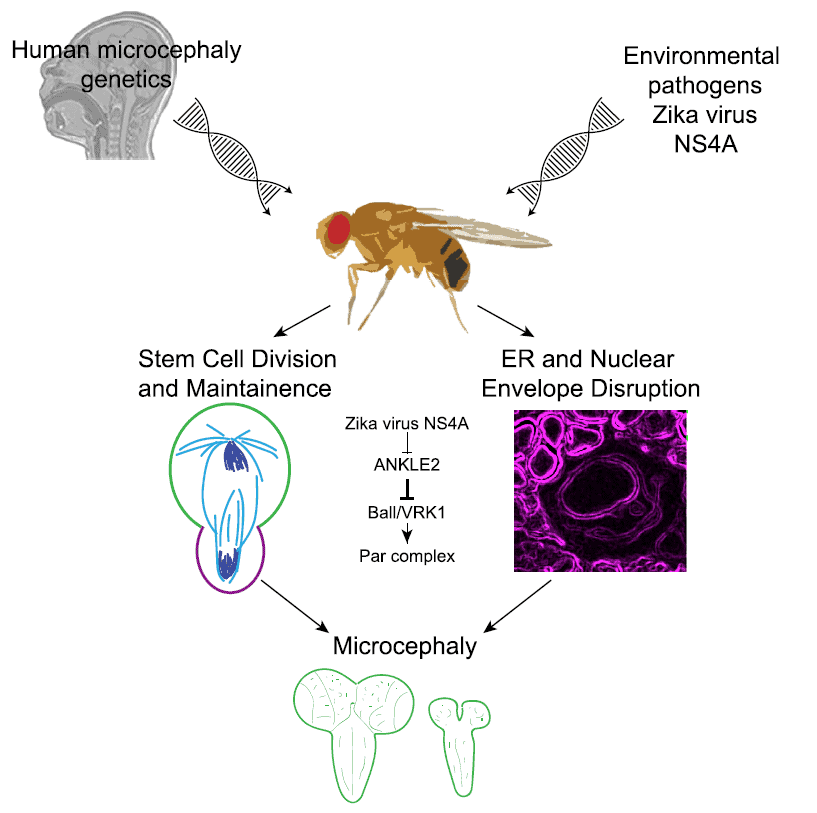
This patient and others in a cohort at CMG had not been infected by Zika virus in utero. They had a genetic defect that caused microcephaly. CMG scientists determined that the ANKLE2 gene was associated with the condition.
Several years ago, Dr. Bellen and colleagues discovered in the fruit fly model that the ANKLE2 gene was associated with neurodevelopmental disorders. In a subsequent fruit fly study, the researchers demonstrated that overexpression of Zika protein NS4A causes microcephaly in the flies by inhibiting the function of ANKLE2, a cell cycle regulator that acts by suppressing the activity of VRK1 protein. Since very little is known about the role of ANKLE2 or VRK1 in brain development, Bellen and his colleagues applied a multidisciplinary approach to tease apart the exact mechanism underlying ANKLE2-associated microcephaly.
The fruit fly helps clarify the mystery
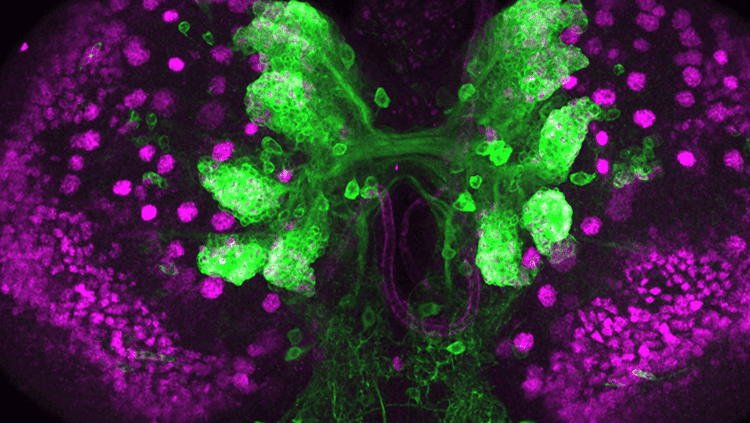
To figure out how Ankle2 mutations were influencing brain formation, the researchers went back to flies. Normally, Ankle2 works with a series of other genes to control the division of neuroblasts — stem cells that give rise to neurons. These cells are crucial for proper brain development.
Mutations in the Ankle2 gene, though, messed with neuroblast division. Larval flies with the mutation had fewer neuroblasts and smaller-than-expected brains. Further analyses revealed more details about how Ankle2 regulates asymmetric neuroblast division. They found that Ankle2 protein interacts with VRK1 kinases, and that Ankle2 mutants alter this interaction in ways that disrupt asymmetric cell division.
The Zika connection
In the future, a drug that protects this protein could stop Zika’s damaging developmental effects, says Dr. Hugo Bellen.
“For decades, researchers have been unsuccessful in finding experimental evidence between defects in asymmetric cell divisions and microcephaly in vertebrate models. The current work makes a giant leap in that direction and provides strong evidence that links a single evolutionarily conserved Ankle2/VRK1 pathway as a regulator of asymmetric division of neuroblasts and microcephaly. Moreover, it shows that irrespective of the nature of the initial triggering event, whether it is a Zika virus infection or congenital mutations, the microcephaly converges on the disruption of Ankle2 and VRK1, making them promising drug targets.”


