Antibody Drug Conjugates (ADCs) have emerged as a promising class of therapeutics in the fight against cancer, offering targeted delivery of cytotoxic payloads to tumor cells while sparing healthy tissues.
Nearly 25 years ago, the FDA approved the first ADCs for treating acute myeloid leukemia. Initially, these early approvals primarily focused on blood cancers. However, today, ADCs have become a formidable treatment option for solid tumors.
Safety is paramount, ensuring the well-being of patients and providing a secure environment in ADC production and administration. Neglecting the toxicity of ADCs and failing to handle them with care can pose serious risks to both patients and professionals involved in their production and administration. But first, what are ADCs exactly?
What are ADCs?
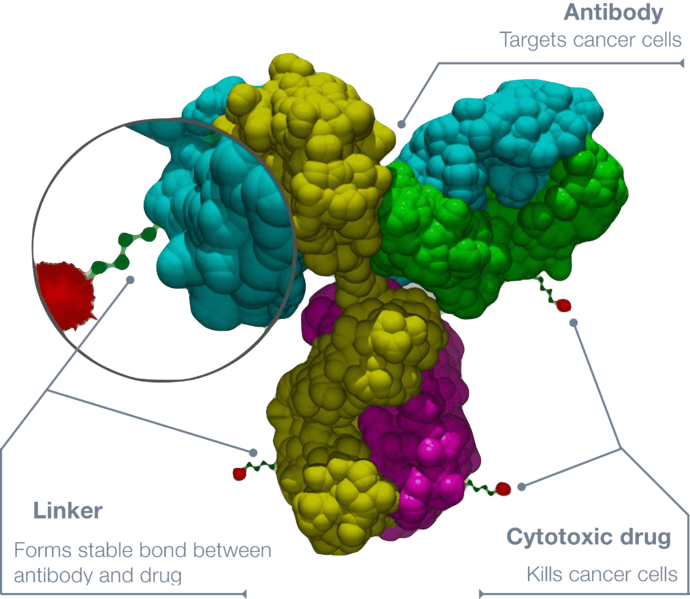
ADCs are a class of targeted cancer therapies that combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs. These drugs are designed to selectively bind to cancer cells, delivering a toxic payload directly to the tumor while sparing healthy tissues.
ADCs have shown promise in treating various types of cancer, offering improved efficacy and reduced side effects compared to traditional chemotherapy.
The Birth of Antibody Drug Conjugates
Early experiments in the 1960s laid the groundwork for using antibodies to deliver cytotoxic drugs to cancer cells. Key milestones, including the development of the first ADCs entering clinical trials in the 1990s, paved the way for subsequent research and innovation.
The first practical application of Antibody Drug Conjugates (ADCs) was gemtuzumab ozogamicin, which received FDA approval in 2000 for the treatment of acute myeloid leukemia. By 2022, ADCs had expanded their usage and were being investigated for potential applications in other diseases.
Several ADCs have achieved remarkable success in clinical trials and have been approved for the treatment of various malignancies. Drugs such as Brentuximab Vedotin and Trastuzumab Emtansine have demonstrated impressive efficacy in Hodgkin lymphoma and HER2-positive breast cancer, respectively, improving patient outcomes and quality of life.
Preclinical and Clinical Development
The journey of ADCs from bench to bedside begins with rigorous preclinical studies aimed at optimizing their design and efficacy. Researchers select target antigens overexpressed on cancer cells and explore various cytotoxic payloads to maximize therapeutic benefit. Additionally, the development of stable linkers capable of releasing the payload within the tumor microenvironment without causing systemic toxicity is of great importance.
The transition from preclinical development to clinical trials represents a critical phase in the validation of ADCs as viable cancer therapies. Phase I trials focus on establishing safety and determining the maximum tolerated dose in human subjects. Subsequent Phase II trials assess the efficacy of ADCs in specific cancer populations, while Phase III trials compare ADCs with standard treatments in large-scale studies.
However, challenges and setbacks, including off-target effects, resistance mechanisms, and manufacturing complexities, underscore the need for continued research and innovation in the field of ADC development.
Challenges and Limitations
The journey of ADC manufacturing is riddled with challenges, foremost among them being the delicate balance between efficacy and safety.
Challenges and setbacks include off-target effects, resistance mechanisms, and manufacturing complexities.
This therapy’s targeted approach hinges on the ADC’s ability to recognize and bind to specific antigens present on the surface of cancer cells. Upon binding, the ADC is internalized by the cancer cell, where the cytotoxic agent is released to induce cell death. However, this can be a double-edged sword.
ADCs are toxic because they are designed to deliver a potent cytotoxic (cell-killing) agent. While this precision reduces the impact on healthy cells compared to traditional chemotherapy, the cytotoxic agents can cause adverse effects if they detach before reaching the target cells or if there is off-target binding.
Moreover, the release of the cytotoxic payload within the body, even in targeted delivery, can lead to side effects due to the potent nature of these drugs. Managing this toxicity requires a delicate balancing act, necessitating careful dosing and monitoring to minimize harm to the patient.
Further challenges arise in the development and manufacturing of these drugs. Ensuring the safety of personnel and the environment requires strict adherence to rigorous manufacturing standards such as current good manufacturing practices (cGMPs).
Scalability further complicates matters. Manufacturers typically implement modular systems and innovative technologies to facilitate the transition from lab-scale to commercial production.
By tackling the manufacturing challenges head-on and safeguarding ADCs’ integrity with specialized, single-use solutions, researchers are set to revolutionize oncology treatment. This approach holds promising prospects for improving the outcomes for patients fighting cancer, heralding a new era of precision medicine in oncology.






