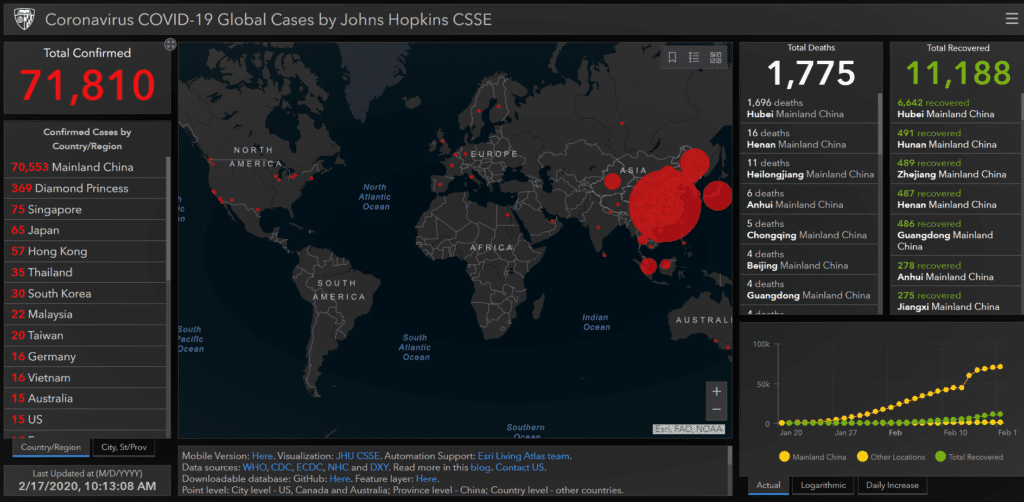
The first anti-viral drug for Coronavirus disease 2019 (COVID–19), also known as 2019-nCoV acute respiratory disease, has been approved for marketing by the National Medical Products Administration since the outbreak. Developed by Zhejiang Hisun Pharmaceutical Company, the drug is expected to play an important role in preventing and treating the outbreak which has now infected 70,553 in China (1,772 deaths), the government said on its official WeChat account.
COVID-19, is an infectious disease caused by SARS-CoV-2 (2019 novel coronavirus), a virus closely related to the SARS virus. The disease was discovered during, and is the cause of, the 2019–20 coronavirus outbreak.
Three potential anti-COVID19 medicines have been officially announced by the Ministry of Science and Technology: Favilavir, Chloroquine Phosphate, and Remdesivir. They all initially showed more obvious curative effects and lower adverse reactions in clinical trials.

Favilavir, formerly known as Fapilavir, an antiviral that has shown efficacy in treating the novel coronavirus, was approved for marketing, the Taizhou government in Zhejiang province announced Sunday.
It is the first anti-novel coronavirus drug that has been approved for marketing by the National Medical Products Administration since the outbreak. Developed by Zhejiang Hisun Pharmaceutical Company, the drug is expected to play an important role in preventing and treating the epidemic, the government said on its official WeChat account.
Chloroquine Phosphate is in a class of drugs called antimalarials and amebicides. It is used to prevent and treat malaria. It is also used to treat amebiasis.
The other one with the most potential – so far – has been Remdesivir, which Gilead had already been developing as a treatment for Ebola disease and Marbug virus infections. It has subsequently also been found to show antiviral activity against other single-stranded RNA viruses such as respiratory syncytial virus, Junin virus, Lassa fever virus, Nipah virus, Hendra virus, and coronaviruses (including MERS and SARS viruses).
Based on success against other coronavirus infections, Gilead provided Remdesivir to physicians that treated an American patient in Snohomish County, Washington infected with 2019-nCoV, and is providing the compound to China, to conduct a pair of trials in infected individuals with and without severe symptoms.
Chinese pharma BrightGene has successfully developed and manufactured copies of the drug, it has also maintained that it will not launch the drug until it has received licensing from Gilead, conducted clinical trials and obtained approval. A study of Remdesivir in COVID-19 has already begun enrolling patients in China, with a Gilead spokesman confirming that two clinical trials will be conducted in Wuhan, the city where the first cases of the novel coronavirus were observed.


