Researchers from the University of North Carolina’s School of Medicine have developed the first high-resolution method of mapping out smoking-induced DNA damage. The technique will help us better understand the risks posed by smoking, how it leads to the appearance of cancers, why some people seem more vulnerable or resistant to cancers, and even how these cancers might be prevented.

The link between smoking, DNA damage, and lung cancers has been well established for decades now, despite what tobacco companies would have you believe. But pinpointing the exact areas of the genome impacted by smoking has so far proved beyond our capabilities, mostly because DNA is really tiny. Now, a team from the UNC School of Medicine have found a way to do just that.
Led by Aziz Sancar, MD, Ph.D., the Sarah Graham Kenan Professor of Biochemistry and Biophysics at UNC’s School of Medicine and 2015 Chemistry Nobel Prize co-laureate, they focused their attention on the major carcinogen in cigarette smoke — benzo[α]pyrene. The team developed a method to map out where this compound ties to DNA by looking at which bits of our genomes undergo repairs following exposure.
“This is a carcinogen that accounts for about 30 percent of the cancer deaths in the United States, and we now have a genome-wide map of the damage it causes,” said Sancar.
“It would be good if this [map] helps raise awareness of how harmful smoking can be,” he added. “It also would be helpful to drug developers if we knew exactly how DNA damage is repaired throughout the entire genome.”
Mind the BaP
For all its villainous effect, Benzo[α]pyrene (BaP) is a surprisingly common substance. It’s part of the polycyclic aromatic family of hydrocarbons (i.e. multi-ringed carbon- and hydrogen- based substances,) which can form from something as innocuous as burning organic compounds all the way to the cold recesses of outer space. They’re so commonplace, in fact, that they’re theorized to have hitched rides on comets and jump-start RNA synthesis, thereby seeding life on Earth.
Image credits Calvero / Wikimedia.
So it might’ve been great for news for life back in the day, but BaPs pose a serious environmental hazard to complex life. Being so common, organisms have evolved to work around BaP at the levels ambient sources (forest fires, a campfire, or an engine) can churn into our environments, but cigarette smoke delivers a huge dose (more than three times the levels of regular smoke) of the compounds directly into our lungs. Not good.
To keep us safe from environmental hazards, our bodies break down toxic compounds into smaller, inert molecules all the time. What happens with BaP is that this process breaks it down into stuff called benzo[α]pyrene diol epoxide (BPDE), which is actually more toxic than the initial hydrocarbon. BPDE chemically ties to DNA, forming a very strong bond (called an adduct) with guanine, one of the four nucleotide bases.
This alters the base enough that it becomes unreadable, so an affected cell won’t be able to synthesize the proteins it encodes or pass on the information to its offspring. All that’s needed for cancer to spring up is for the gene in question to be a tumor suppressor gene, for example. Lab trials with mice have shown that even a moderate dose of BaP applied to the animals’ skin would virtually guarantee the appearance of tumors. That’s why BaP and BPDE, via smoking, are considered to be the most important cause and multiplier factors for lung cancers.
DNA re-formatting
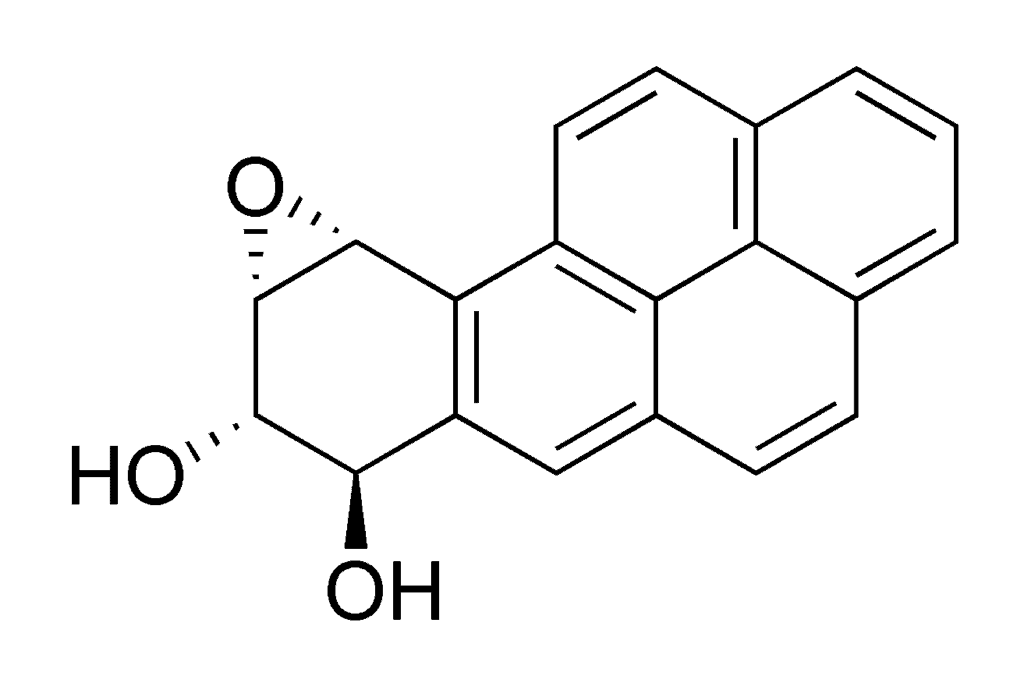
Image in Public Domain, via Wikimedia.
Keeping an eye on the whole genome to see where this process takes place would be impossible. So what the researchers did instead was to scour the cell for the affected strands, which would simply float around to be recycled during the repair process. These bits of code were tagged chemically, retrieved, and sequenced. Fitting all the sequences together gave the team a map of all the spots where DNA was damaged by smoking and currently under damage.
“This new method can be applied to any type of DNA damage that involves nucleotide excision repair,” Sancar said.
Still, given the expenditure and sheer workload that goes into DNA sequencing, the proof-of-concept map the team has published doesn’t have the best resolution possible. But it does show that such maps, which can help understand the relationship between disease and DNA damage, are feasible. In the future, they could help uncover what dose of a particular substance can overwhelm our natural ability of nucleotide repair, which genes or gene variants help promote DNA repair, and if there are any particular areas where the damage is harder to repair.
From their prototype map, the team was able to show that BPDE-related repairs tend to occur more often when the guanine it ties to is next to a cytosine, rather than a thymine or adenine — suggesting these areas are high-risk or “hotspot” areas for BPDE-induced mutations.
“I’m certain,” added Sancar, “that all this information will lead to a better understanding of why certain people are predisposed to cancer, and which smoking-related mutations lead to lung cancer specifically.”
Sancar also hopes that their highly specific evidence of smoking’s harm at the cellular level might convince some smokers to kick the habit. There are about 40 million smokers in the United States and over a billion worldwide.
The paper “Human genome-wide repair map of DNA damage caused by the cigarette smoke carcinogen benzo[a]pyrene” has been published in the journal PNAS.






