To say that 2020 has revolved around the pandemic would probably be quite true. But 2021 is shaping up to be all about our answer to the virus, in the form of a vaccine. At least one vaccine has been approved for use in the EU and, although there are many candidates in the works, none of these were developed in what you’d call a ‘traditional’ timeline. We’ve had to wait a whole year, but that’s still a record-breaking speed for this class of substances.
So let’s take a look at this pace of development. How exactly was it that the vaccine got developed so quickly? Does it say anything about how reliable the compound is? Can we trust it?
How vaccines are made
First of all, we should take a brief view on how vaccines are produced so we have a good idea of the process we’ll be discussing today.
A vaccine basically works by giving our bodies the opportunity to see and study a viral threat in a controlled manner. This experience lets it develop its own biochemical weapons against the virus (which are much, much better than our pharmacological tools for the job). In very broad lines, there are four ways to produce such a substance:
- Viral attenuation. This involves altering a virus’s structure or genes in such a way that it becomes hard for it to replicate. This uses a technique called cell culture adaptation, which involves re-adapting the virus to grow in specialized cells inside the lab, and not in the normal cells they’d meet in our body. While impaired, there’s still a small chance that such viruses can replicate inside the body, which is why they’re referred to as ‘live’, ‘weakened’, or ‘attenuated’ viruses. The measles, mumps, rubella, and varicella vaccines are made this way.
- Destruction of the viral genes. This is safer than the previous approach to an extent, in the sense that a virus’s genes are completely destroyed so it can’t replicate — these are referred to as ‘killed’ vaccines. A virus’s genetic material is exposed to the chemical formaldehyde during the process, which destroys them permanently. The polio vaccine is produced this way.
- Physical breakdown of the pathogen. In this case, the virus or bacterium in question is physically broken apart, and certain elements of it are then used to produce the vaccine. This is also a very safe approach as there isn’t any genetic material in the vaccine, so there’s no way for an infection to set in. Still, from studying the bits that do make it into the final vaccine, our immune system learns how to spot and then attack the threat. The vaccine against hepatitis B is produced this way.
- Toxoid vaccines. For those pathogens that don’t directly make their host sick, but use toxins (weaponized proteins) to do it, we have toxoid vaccines. Bacteria like diphtheria or tetanus function this way. To make a vaccine against them, the toxins are isolated, purified, and then chemically neutralized. Toxoid vaccines also don’t pose any risk of infection as they carry no genetic material.
Typically, the process of developing a vaccine takes 10 to 15 years on average. Obviously, this process used to take a lot longer before, but we’re getting better at it, thanks to our modern know-how and technology. Apart from the COVID-19 one, the fastest-developed vaccine in history (from the time the virus was isolated to the finished product) was the mumps vaccine in 1967, which took 4 years. In contrast, the influenza virus was first isolated in 1933, but an effective vaccine was only licensed in 1945.
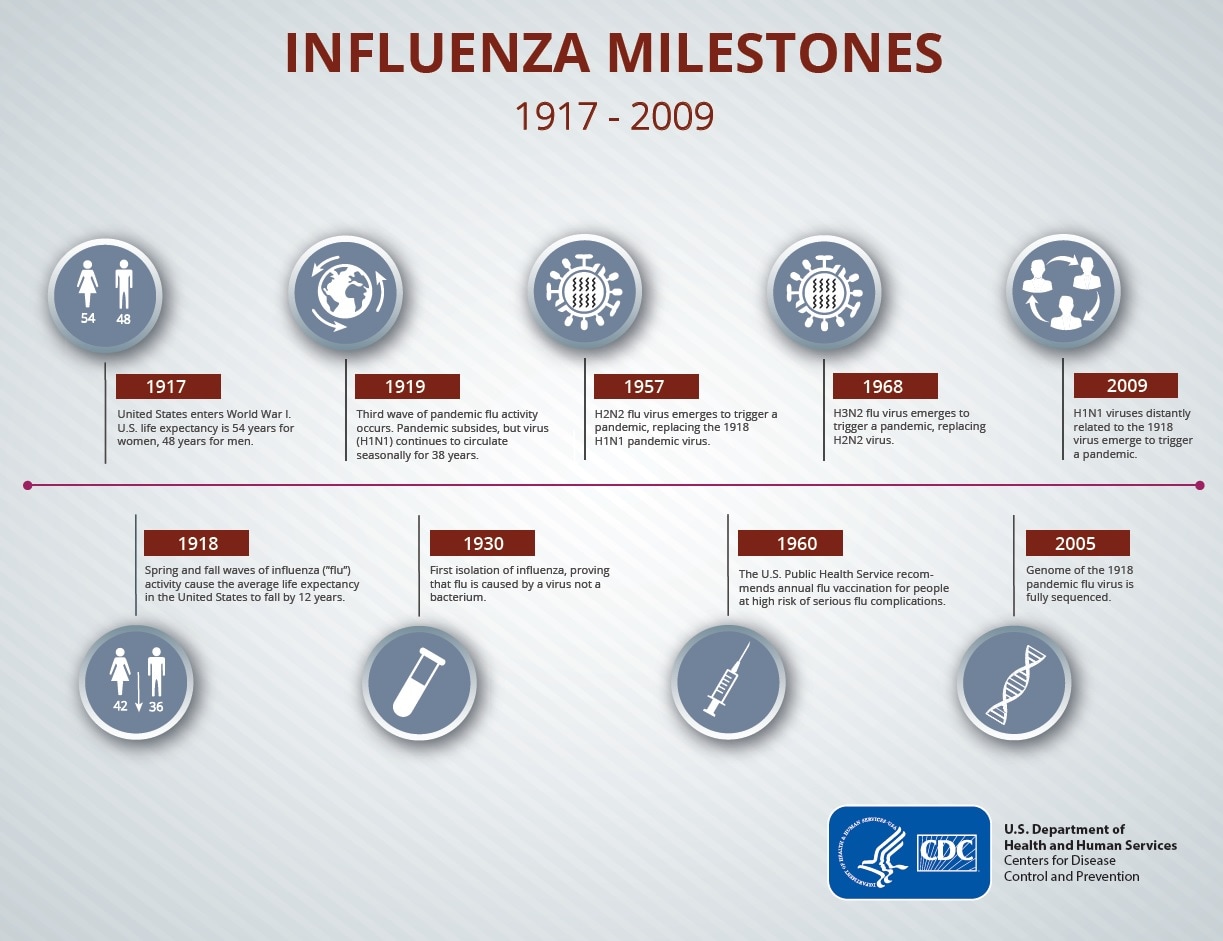
So that’s our reference timeframe for the development of a vaccine. During this time, researchers have to isolate the threat, decide on the best approach for turning it into a vaccine and then go about it, and test their compound on every step of the way. Human trials tend to be the most visible steps of vaccine development, but they’re the tail end of this process; extremely few candidates make it this far into development.
Those who wonder why we didn’t have it sooner should keep in mind that this is the fastest-ever developed vaccine. It might seem like a long time when you’re stuck inside waiting for it, but in relative terms, we got it blazingly fast.
Wasn’t that too fast?
Here, then, comes the other side of the coin. I sometimes get asked regarding the safety of the vaccine after explaining that it’s been developed at breakneck speeds — surely, then, some corners had to be cut? Well, not really. All the data we have available for the two most promising vaccines (the Pfizer and Moderna ones) show that both are highly effective and quite safe. While the development process was streamlined as much as possible, critical steps such as testing for side-effects were not skipped or cut short. So, then, how did we pull it off?
Well, governments around the world did step up and do their best to help bring a vaccine to completion (such as the US’ Operation Warp Speed). The fact that the world was facing a scary, ruthless, and highly-contagious virus probably also helped light a fire under all kinds of people involved in the process, too.
Researchers as well as the public understood the need for such a vaccine, so volunteers stepped up quickly to help test it. Each vaccine candidate goes through three steps, or ‘phases’, of human trials. These are meant to determine if they are safe to use, and how best to do so. Phase 1 checks for side effects using a small number of participants, generally 30 at most. It starts with some of them receiving a small dosage, which increases in subsequent groups if everything goes well. Phase 2 determines what quantity of the vaccine to use for best effects, and Phase 3 compares its safety and efficacy to the current standard treatment. Phase 3 also typically employs placebos for more accurate results.
The latter two steps use more participants, between 25 to 100 for Phase 2 and several hundred or thousands for Phase 3. Moderna’s Phase 3 trial ran with “over 30,000 participants in the U.S”, while Pfizer’s trial included some 43,500 people, which is quite sizeable. The trials tracked the candidate vaccines’ efficacy over time and any side-effects. Both found these to be mild to moderate, “short-lived”, and “generally resolved within two days”. Apart from a low incidence of fever in the Pfizer trial (16% for younger and 11% for older volunteers), these side-effects seem to typically be mild rather than worrying.
Data from current vaccination campaigns in Europe and the U.S. support these results, with relatively few cases of severe reactions to the vaccine.
Well if they didn’t cut any corners in the safety department, how did they pull it off so fast? By far the largest deciding factor was financial.
Money matters

We like to pick on Big Pharma for profiteering from people’s misfortunes. To an extent I definitely agree and join in on that — nobody should have to pay to avoid suffering, disease, disability, or death. It’s revolting when profits become more important than the lives this industry should protect.
But at the same time, we have to face the truth. No matter how well-intentioned a drug company is, it can only function as long as it can pay for it. It has to play within the rules of the systems that are already set in place, and for now, that means they need to turn a profit or they’re toast.
Historically speaking, vaccines have not been profitable for either the pharmaceutical industry nor medical professionals. A study published in 2009 found that “the variable costs of vaccine administration exceeded reimbursement from some insurers and health plans” for medical personnel, meaning that your doctor is probably losing money for every vaccine they administer. A darker way to look at it is that vaccines cost the healthcare sector twice — once in the cost of development, production, and administering it, and then in the profits they lose in the long term from preventing disease.
So as callous as it sounds, it does very much come down to money. Vaccine development is expensive. For every candidate that reaches the human trial phase, countless others were scrapped along the way (after heavy expense). Furthermore, vaccines are a risky product. Once a company has developed a safe, efficient vaccine, it needs further investment to ramp up production capabilities and storage sites (which often need to be refrigerated, so they incur a high running cost). The doses need to be stored until needed, which could happen tomorrow or 20 years from now. They might even go bad before they’re needed.
All of this adds up to make vaccines, in general, a very expensive and risky investment for producers. In the case of the COVID-19 vaccine, one thing that helped tremendously was that governments around the world came to shoulder some of that risk. In the US, for example (although they are not at all the only country to do so), the government moved huge sums of money to guarantee to pharmaceutical companies that any successful vaccine candidate would be bank-rolled for production. Contracts were also signed ahead of time for millions of doses.
Keep that in mind. Without even knowing if a vaccine will work or not, governments guaranteed that they would pay for its production and that they would buy a pre-known (and significant) quantity of doses. This essentially removed the risk from the equation: producers knew that their vaccine would be paid for and the minimum quantity of doses they would sell ahead of time. If you’re familiar with the whole Steam vs. Epic Games situation, you know how attractive this business model can be from the producers’ side.
Assured that they won’t bankrupt themselves, pharmaceutical companies started work on the vaccine extremely quickly. In March, five days after the WHO declared a global pandemic, Moderna was already starting safety trials for its vaccine candidate. In the end both them and Pfitzer used this opportunity to develop what was previously a theoretical vaccine production method — mRNA vaccination.
Instead of relying on traditional vaccination approaches (due to time constraints and the associated risk of using live viruses in the case of COVID-19), an mRNA vaccine uses messenger RNA (the counterpart of DNA) to teach our bodies about the virus. Researchers first isolated the part of the viral genome that encodes its spike proteins — these are the ‘keys’ the virus uses to enter our cells — and copied it into a messenger RNA strand. Our cells’ own molecular machinery then turns this into finished spike proteins, and from here on it works like any other vaccine.
Its biggest advantage is also its largest disadvantage: how unstable RNA molecules are inside our body. It makes the delivery of the vaccine way more complicated (doses need to be kept at such low temperatures to prevent the mRNA from breaking down), but it also means that any side-effects set in and are overcome quickly.
All this wouldn’t have happened if governments (and thus, society) hadn’t taken the risk off the hands of those developing the vaccine. It’s a good reminder that while the chase for profits can damage the greater good, companies have no option but to ensure they remain profitable — sometimes, making sure they have the money they need is to everyone’s benefit. It’s also a reminder that we have the ability to solve many of the world’s problems if only we were to shoulder the financial cost.






