Black silicon, a novel nanosurface reminiscent of a bed of nails, is the worst place to find yourself in if you’re a bacteria — but one of the best if you’re a respectable, hard working eukaryote cell. The surface has been shown to discourage bacterial growth while leaving our cells unharmed, a property in high demand as more patients require catheters and implants.
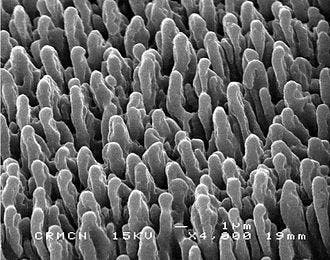
Image credits Sedao/Wikimedia
Once an implant is grafted into the human body, it starts a feeding frenzy of human and bacterial cells, each trying to colonize as much of the surface as possible. Needless to say, you don’t want bacteria to colonize anything, ever, in your body (with a few exceptions.) Thus, surfaces that stack the playing field in favor of our cells would be ideal as coatings for such objects.
An international team of researchers has developed a nanomaterial that does just that. Not ones for subtlety, they created the nano-equivalent of a spike pit, making it on forever. Called black silicon, the surface’s nanotopology physically rips apart bacteria while leaving human cells alone.
The team drew inspiration from previous research on dragonfly wings, which are built from bactericidal nanostructures. Black silicone has similar bactericidal properties — zoom in enough, and it’s remarkably similar to a bed of nails. Because bacteria are smaller than our (eukaryotic) cells, the pressure these points exert on their membranes should break them apart while leaving larger cells (which can distribute their weight on more points) unharmed. That’s the theory at least.
To test their material, the team coated surfaces with either black (bSi) or regular silicon (Si,) and infected them with either Pseudomonas aeruginosa or Staphylococcus aureus, both human pathogens. They then added cells harvested from monkey kidneys (called COS-7 cells) to see if the black silicon has any effect on the pathogens.
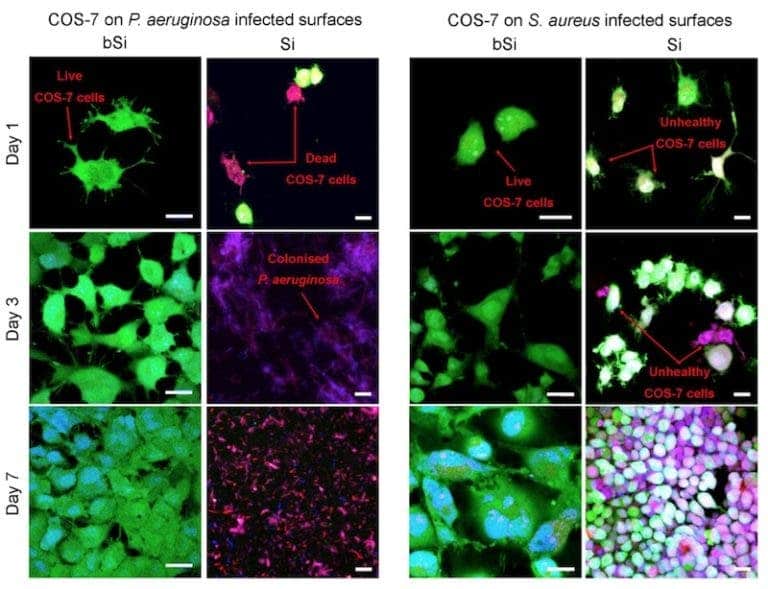
The monkey cells (colored in green) had a lot of trouble with the bacteria in the absence of black silicone — P. aeruginsoa completely prevented any cells from growing on the regular silicone surface, while S. aureus slowed their growth rate significantly. But on the black silicon surface, the bacteria were killed and COS-7 cells developed nicely. This proves that the surface is able to kill both Gram-positive (thick cell wall, represented by S. aureus) and Gram-negative (two outer membranes, represented by P. aeruginosa) bacteria. Maybe it can even help with drug-immune bacteria.
It also doesn’t seem to cause any problems for eukaryotic cells. Microscopy observations showed that the cells simply deform their membranes around the tiny spikes, harmlessly engulfing them. When injected into mice, black silicon didn’t trigger any adverse effects such as an inflammatory response.
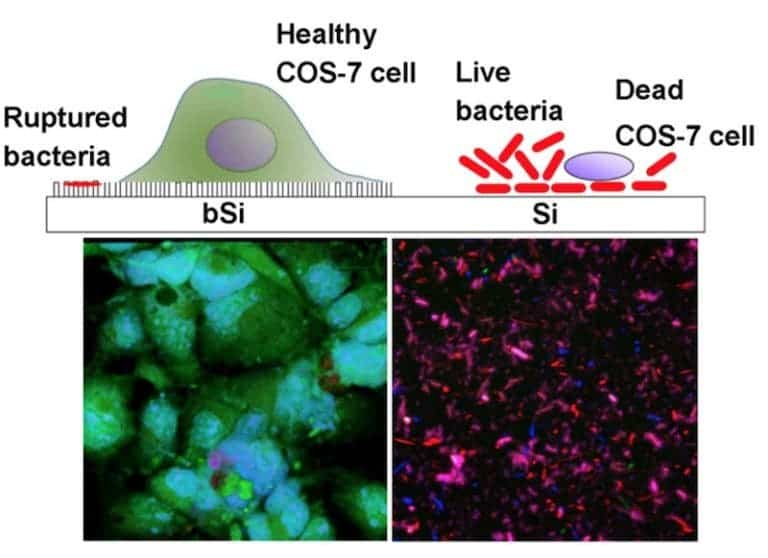
The authors say that this is the first time anyone has proven that eukaryotic cells can grow on a surface previously contaminated with deadly bacteria. They’re also hoping to have their new material read for commercial purposes soon.
And I for one am looking forward to it, because there’s something really metal about impaling bacteria to death.
The full paper, “‘Race for the surface’: eukaryotic cells can win,” has been published in the journal Applied Material Interfaces.






