
Computer chips can be found in just about anything nowadays. They’re in the device you’re using right now to read this article, and dozens more can be found in your vehicle, helping regulate engine temperature and stabilize suspension systems. You’ll find chips in WiFi routers, refrigerators, and even in toasters. Just about anything that you plug into a socket now carries a tiny computer inside.
The fact of the matter is that the modern world runs on computer chips, but they can also eat up a lot of rare minerals and especially precious metals like gold and platinum — and recycling them is no easy task. Looking to address this challenge, researchers at Nagoya University in collaboration with the Tokyo Institute of Technology have discovered an unlikely solution in the form of Prussian blue, the world’s first synthetic pigment that forever changed the art of painting.
If you stacked the contents of one ton of mobile phones, you’d find up to 400 grams of gold inside. That might not sound like a lot, but it’s still up to 80 times more than you’d find in one ton of natural ore. With the right technology to extract all those tiny amounts of metal from each device, it could be much more cost-effective to recycle gold and platinum from electronic waste than to mine them. Gold mining is also one of the most destructive industries in the world, which can contaminate drinking water and soil, and destroy pristine environments due to mercury and cyanide pollution, endangering the health of people and ecosystems.
From Picasso to the waste processing plant
Before Prussian Blue was invented by accident by German painter Johann Jacob Diesbach at the turn of the 18th century, painters had to use only naturally occurring pigments, such as indigo dye or the highly expensive ultramarine made from lapis lazuli to make deep-blue hues. Armed with a new chemistry recipe, Diesbach was able to now produce virtually unlimited quantities of Prussian Blue. Other chemists then started producing other synthetic pigments, making them much more affordable and transforming art.
You can find Prussian Blue in many masterpieces, such as Vincent van Gogh’s The Starry Night or Hokusai’s The Great Wave off Kanagawa. During his famous Blue period, Picasso used Prussian blue virtually in every one of his acclaimed works.
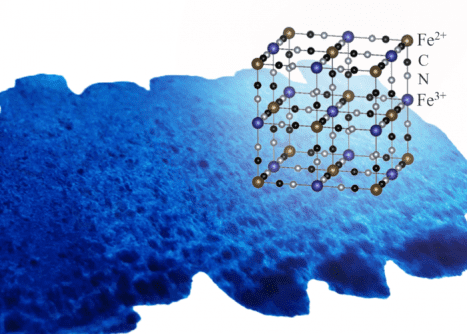
Today, the pigment has fallen out of favor among modern painters, who have better options at their disposal. However, Prussian blue is still being used for two very different purposes. Pills containing Prussian blue are often used in emergency medical situations to treat heavy metal poisoning. The pigment forms a jungle-gym-shape lattice with nanometer-sized gaps that is ideal for trapping individual metal ions, including toxic metals like thallium or radioactive cesium.
Prussian blue was also used extensively in the aftermath of the 2011 tsunami that caused the nuclear accident at the Fukushima plant, sucking up radioactive cesium from the soil in the vicinity of the disaster site. This is how the Japanese researchers got the idea to investigate the pigment’s ability to recover metals from electronic waste.
Previously, the team led by Jun Onoe, Professor of Engineering at Nagoya University, showed that Prussian Blue pigments could trap rare metals like molybdenum, as well as ruthenium, rhodium, and palladium, among other metals from the platinum group on the periodic table.
In their new study, Onoe and colleagues used X-rays and ultraviolet spectroscopy to learn more about how the pigment performs these feats.
“I was surprised to discover that Prussian blue uptakes the platinum-group precious metals by substitution with iron ions in the framework while keeping the jungle-gym structure,” Professor Onoe explained, adding that this mechanism allows the pigment to uptake more gold and platinum metals than any other biobased absorbent.
Besides helping to solve our growing electronic waste problem, Prussian blue could also be a solution for nuclear waste, whose disposal is incredibly complex and risk-prone. Spent nuclear fuel rods are typically reprocessed and converted into a glass-like state for long-term storage in geologically-insulated facilities, sometimes miles-deep underground ‘nuclear tombs’. During this process, platinum-group metals can settle on the sidewall of the melter used in the nuclear waste reprocessing plant, which needs to be flushed to preserve the stability of the vitrified objects. Prussian blue could be an easy, cheap fix in this case.
“Our findings demonstrate that Prussian blue or its analogues are a candidate for improving the recycling of precious metals from nuclear and electronic wastes,” says Professor Onoe. “Especially when compared to conventionally used bio-based adsorbents/activated carbons.”
From the painter’s palette to electronic and nuclear waste cleanup, Prussian blue has been quite the ride so far.
The findings appeared in the journal Scientific Reports.


