Water in seas and oceans seems blue, but a cup of water seems perfectly transparent. Why is that? The hue of water is caused by the chemistry of water molecules. Simply speaking, the molecules absorb light towards the red part of the light spectrum, leaving the blue parts available for us to see. But the real answer is a bit more complex.
You’ve heard it since you were a kid — water doesn’t have a taste, a smell, or a color. But as science goes to show, that’s not quite right. While relatively small quantities of water appear to be colorless, water’s tint becomes bluer and bluer as its thickness increases.
Blue water
You’ve seen it in deep oceans — water becomes blue. This is not to do with any biological element (like water in a lake seeming green because of algae, for instance). It’s only got to do with the chemistry of water.
Sure, one could argue, but that’s only because the water is reflecting the color of the sky. That’s seemingly a reasonable argument, but this only happens at relatively low angles and when the water is smooth. In the vast majority of cases, the sky doesn’t play an important role in this. Even in indoor swimming pools where the sky is not visible, water can appear blue (or rather, cyan).
In fact, as research has shown, a slight blue hue is an intrinsic property of water (in and of itself).

The light turquoise color is caused by weak absorption in the red part of the visible spectrum. The color becomes visible when looking at a white light source through a long pipe (or something else) filled with pure water and closed at both ends with transparent glass.
Why does it do this? The answer (as is often the case with water) is not that straightforward.
The color spectrum
In the 1660s, English physicist and mathematician Isaac Newton started a series of curious experiments. Newton was experimenting with sunlight and prisms, and before too long, he found a remarkable property of light. Newton showed that light, or what we normally call light, is actually composed of seven visible colors. These colors are the colors of the rainbow — because the rainbow itself shows up as a spectrum.
The colors in the spectrum correspond to different wavelengths of light.
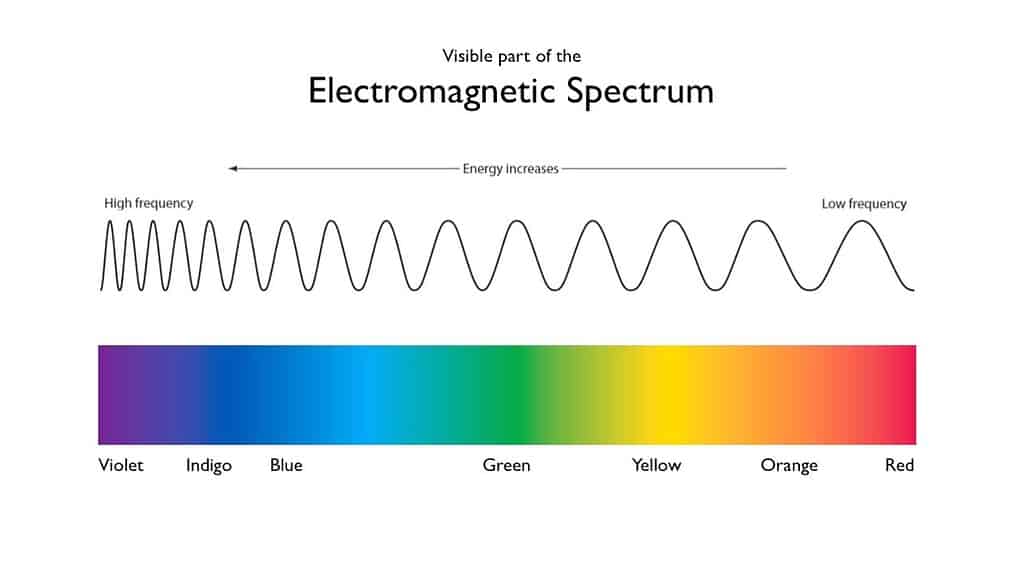
This also explains what makes things colored. Different objects absorb some wavelengths more than others. The wavelengths that the objects reflect back more are the color of that object. Things can get more complicated, but that’s the essential part.
So the colors towards the red site of the spectrum are absorbed. The remaining light seen is composed of green, cyan, and blue wavelengths. This is the main reason the ocean’s color is cyan or blue.
Water absorbs fewer blue wavelengths, reflecting them back — hence why water is blue. But that only seems to work for large bodies of water. Why is water in a glass transparent?
Color of water
There’s a simple, reasonable explanation for why water is blue in the ocean but not in a cup.
In small quantities, like a glass of water, this absorption process is hardly noticeable. The light doesn’t travel far enough to let the blue triumph over other colors. However, in larger bodies of water, like an inviting swimming pool or the deep expanse of the ocean, the path of light is long enough that the magic unfolds before our eyes, revealing water’s true colors.
But that explanation doesn’t really tell the whole story.
Water is weird, in more than one way. Amongst its unique properties, the way it absorbs some photons is also unparalleled.
The photons that water absorbs promote transitions to high overtone and combination states of the nuclear motions of the molecule, i.e. to highly excited vibrations. From what we know, water is the only substance in the natural world that exhibits a hue caused by excited vibrations — other materials owing their colors to the interaction of visible light with the electrons.
The particularities of water color are also brilliantly illustrated in glacial ice.
Small amounts of ice appear white/transparent for two reasons. Firstly, there are a lot of air bubbles, which makes it seem white. Secondly, like in the case of the water glass, it’s a small quantity of water, that makes it seem more transparent. But glaciers are large bodies of ice, and they’re so big they have significant pressure. This pressure causes the air bubbles to be squeezed out. This is why large masses of ice also appear cyan.
Apparent and real color
We’re talking about water as if it’s just two molecules of hydrogen and one molecule of oxygen. But that only happens in chemistry books and labs. In the real world, water is never just water — it always contains something else in addition.
For instance, if you look at an ocean, you’re likely to see some bluer or greener patches. This has a lot to do with the scattering of particles in that part of the water. This scattering skews the color from cyan to different shapes.
Simply put, in real life, you’re never going to find pure water. In a swimming pool for example, which has other chemicals that make it more sanitary, water will also appear blue, even indoor, where there is no sky to be reflected. For this reason, in practice, water color is split into two: apparent color (the color as we initially see it), and real color (after it has been filtered and purified) — which is light blue/cyan.
Ultimately, despite being the substance that nourishes life on Earth, water is also a mysterious substance. Its color, like many of its physical properties, is a tapestry of complex physics and chemistry. The color of water is a story written in the language of wavelengths and absorption, narrated by the physics of light and the unique chemistry of water molecules.







