Limestone is a common type of rock in geology. It has a rich history in geological research and a wide array of uses that extend from construction to agriculture. Let’s dive deeper.
What is Limestone?
Limestone is a type of carbonate sedimentary rock primarily composed of calcium carbonate (CaCO3). It typically comprises two different minerals: calcite and aragonite, which have the same chemical formula but different types of crystals.
Limestone forms through both chemical and biological processes, including the accumulation of sea creatures‘ shells and corals. In fact, these rocks often come from organic sources.
The Formation and Composition of Limestone
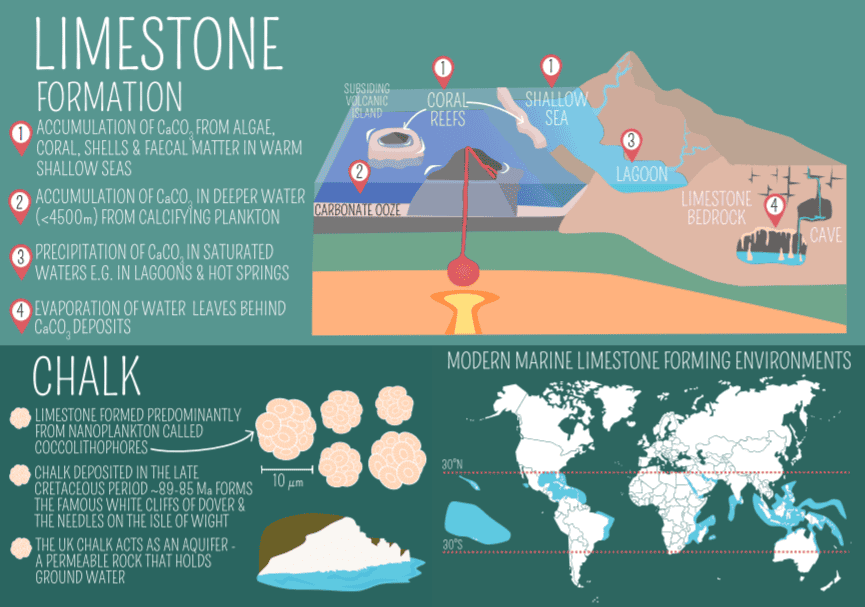
Limestone’s journey from a simple sediment to a sedimentary rock is a fascinating story of Earth’s geological processes. This process, taking place over millions of years, usually involves a combination of biological, chemical, and physical factors.
Biological Formation
Many marine organisms, such as corals, foraminifera, and mollusks have shells or structures made of calcium carbonate. The skeletal remains of these creatures can accumulate and fossilize on the ocean floor. Over time, these layers of biological debris get compacted and cemented to form limestone. This biological process has been a primary contributor to limestone formation for the last 540 million years. Yes, some of the limestone mountains and hills were created thanks to biological (and geological) activity.
Chemical Precipitation
Apart from biological accumulation, limestone can also form through the direct chemical precipitation of calcium carbonate from marine or fresh water. This process can occur in warm, shallow seas and freshwater environments like lakes and rivers. Limestone is chemically pretty pure. The chemical formula for this process can be simplified as:
Ca2+(aq) + 2HCO3−(aq) → CaCO3(s) + H2O(l) + CO2(g)Environmental Conditions
The environmental conditions under which limestone forms are crucial. Warm, tropical seas are particularly conducive to limestone formation due to their abundant life and the ability of warm water to hold less CO2, which aids in the precipitation of calcium carbonate. Additionally, the depth of water plays a role; most limestone originates in shallow, calm marine environments like continental shelves.
Diagenesis
Diagenesis is the process that turns loose sediments into solid rock. In the case of limestone, this involves further compaction and cementation of the calcium carbonate sediment. Over time, pressure from overlying sediments, along with chemical changes, solidify these layers into hard limestone.
Variations in Composition
While predominantly composed of calcium carbonate, limestone can also contain varying amounts of other materials like clay, silt, sand, and organic matter. These impurities can affect the color and texture of the limestone. For example, the presence of iron oxide can give limestone a reddish hue, while organic matter can lead to darker shades.
Different types of limestone form under different conditions and environments. For instance, oolitic limestone, composed of small spherical grains called ooids, forms in warm, shallow, wave-agitated waters. Chalk, a soft, white form of limestone, is made from the microscopic skeletons of marine plankton.
Types of Limestone and Classification
The classification of limestone is based on several factors, including its formation process, texture, and composition. Two widely recognized classification systems, the Folk and the Dunham, help in understanding these types.
Folk Classification
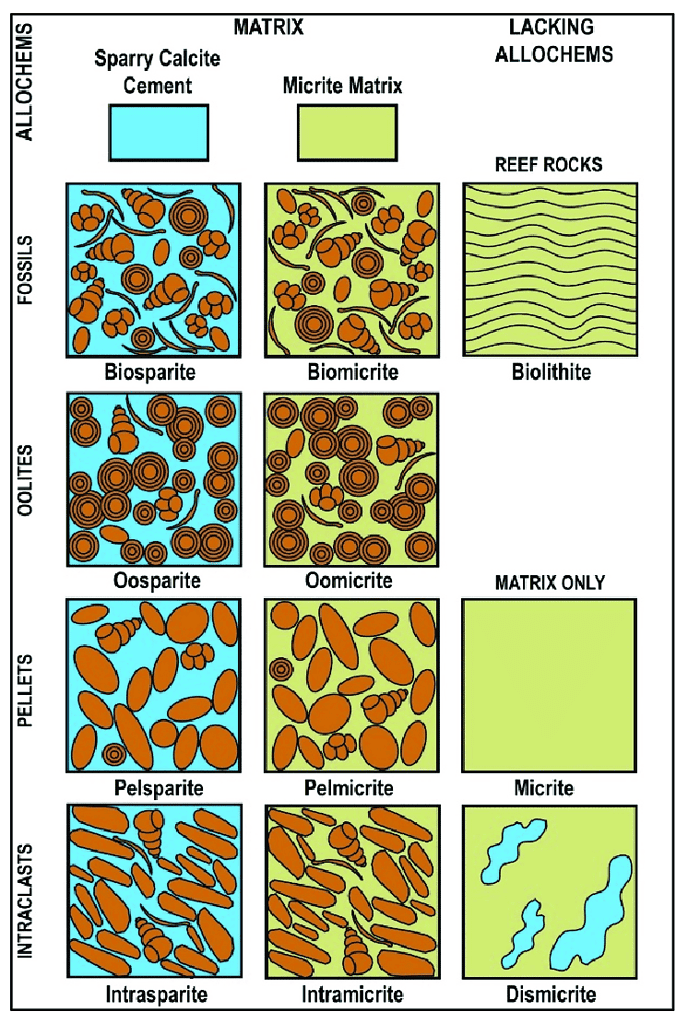
Developed by Robert L. Folk, this classification system focuses on the detailed composition of grains (allochems) and the interstitial material in carbonate rocks. It uses a two-part naming system:
- Allochem Type: Indicates the dominant grain type, such as ooids in oolitic limestone or shell fragments in fossiliferous limestone.
- Matrix Type: Refers to the nature of the interstitial material, like micrite (fine-grained calcite) or sparite (crystalline calcite).
For example, limestone primarily composed of ooids with a crystalline matrix is termed an “oosparite.”
Dunham Classification
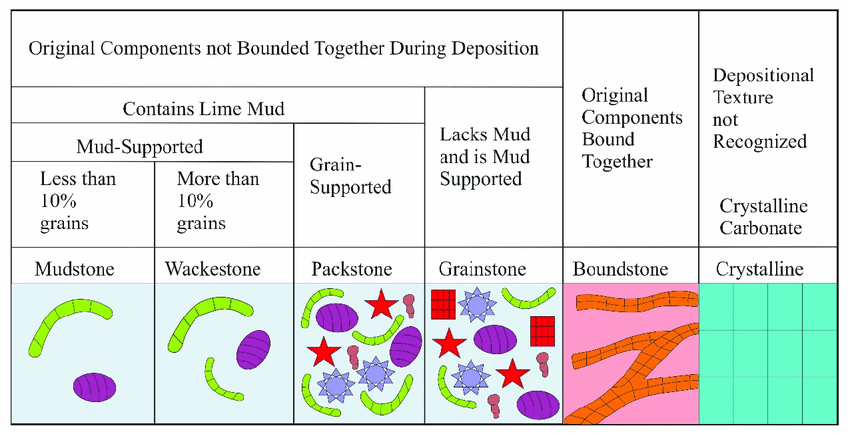
Introduced by Robert J. Dunham, this classification focuses on the textural features of the rock and its original porosity. It categorizes limestone into:
- Mudstone: Predominantly micritic with no grains.
- Wackestone: More than 10% grains but still supported by a micritic matrix.
- Packstone: Grain-supported structure with no micritic matrix.
- Grainstone: Entirely grain-supported with no matrix.
- Boundstone: Formed by in situ growth of organisms, like corals.
Notable Types of Limestone
Travertine

A type of limestone formed by mineral springs, especially hot springs. It is characterized by a fibrous or concentric appearance and is used extensively in architecture.
Chalk

Composed mainly of the mineral calcite, this soft, white, porous limestone forms from the skeletal remains of microorganisms like foraminifera. It’s known for its fine grain and typically forms in deep marine environments.
Tufa

Tufa towers are tall columns of limestone that form in lakes through chemical reaction of spring water with saline lake water.
Similar to travertine but forms in freshwater environments. Tufa is less dense and has a more porous texture compared to travertine.
Oolitic Limestone

Characterized by small, spherical grains called ooids, this limestone forms in shallow, warm, marine waters, indicative of a high-energy environment.
Fossiliferous Limestone

Contains visible fossils, offering significant insights into past life forms and environments. It’s important for paleontological studies.
Lithographic Limestone

A fine-grained, dense limestone formed in deeper water, known for preserving detailed fossils and used historically in lithographic printing.
Characteristics of Limestone
Limestone exhibits a range of characteristics that not only define its appearance and feel but also hint at its formation history and environment. These characteristics make limestone a fascinating subject for study and a valuable resource in various applications.
Physical Properties
- Color: Limestone typically presents a color palette ranging from white to gray. However, it can also display colors like yellow, red, or black, influenced by impurities such as iron oxide or organic matter.
- Texture: The texture of limestone can vary significantly, from fine-grained to clastic. It might be smooth and uniform or rugged with visible fossil fragments or grain structures.
- Hardness and Strength: Generally, limestone is a soft rock with a Mohs hardness ranging from 2 to 4. Despite this softness, some denser forms of limestone exhibit considerable strength and durability.
- Density and Porosity: The density of limestone varies depending on its porosity, which can range widely. This affects not only the weight but also the strength and weathering behavior of the rock.
Chemical Properties
- Reactivity with Acid: A hallmark of limestone is its vigorous reaction with dilute hydrochloric acid, releasing carbon dioxide gas. This property is a key identifier for limestone and is due to its high calcium carbonate content.
- Composition Variability: While primarily composed of calcium carbonate, limestone can also contain varying amounts of other minerals and impurities, influencing its reactivity, color, and texture.
Structural Features
- Layering and Bedding: Many limestone formations exhibit distinct layers or bedding, reflecting changes in environmental conditions over time.
- Fossils: Limestone often contains fossils, which can range from microscopic remains of marine organisms to larger skeletal fragments. These fossils are not only important for understanding past life but also for correlating the age of different limestone layers.
Weathering Patterns
Limestone weathers in unique ways due to its solubility in weak acids. This leads to the formation of characteristic landscapes like karst topography, with features such as sinkholes, caves, and underground streams.
Thermal Properties
Limestone has good heat resistance and thermal conductivity, which makes it a preferred material in construction for flooring, roofing, and other applications where temperature moderation is essential.
Distribution and Occurrence of Limestone

Limestone is found in a variety of geographical and geological settings. Its widespread distribution and occurrence are closely tied to the Earth’s history and the evolution of life, particularly in marine environments.
A significant proportion of the world’s limestone originates from shallow, warm marine waters, particularly around continental shelves and platforms. These areas, teeming with marine life, are ideal for the accumulation of calcium carbonate, the primary constituent of limestone.
Karst landscapes, another hallmark of limestone’s presence, are characterized by distinctive features like sinkholes, caves, and underground drainage systems. The Burren in Ireland, the karst terrain in Guilin, China, and the Yucatán Peninsula in Mexico stand as prime examples of such topographies shaped over millennia by limestone.
Beyond the oceans, limestone makes a prominent appearance in mountain ranges, having been uplifted from ancient seabeds through tectonic activities. The Dolomites in Italy and parts of the Rocky Mountains in North America are notable examples where limestone has been thrust to prominence.
Significant geological sites further demonstrate limestone’s wide reach and importance. The White Cliffs of Dover in the United Kingdom, composed predominantly of chalk, are iconic for their sheer cliffs teeming with microorganism remains. The Great Barrier Reef, although primarily known for its coral, is partially composed of limestone accumulated over thousands of years. In Turkey, Pamukkale’s stunning terraces, formed from travertine, a type of freshwater limestone, exhibit another aspect of limestone’s versatility.

Geologically, limestone has marked various eras, notably the Paleozoic and Mesozoic. The Paleozoic era, especially during the Carboniferous period, saw extensive limestone formations that contribute significantly to today’s coal reserves. The Mesozoic era, particularly in the Jurassic period, was marked by the formation of vast chalk beds and other limestone types, leaving a rich legacy that transcends time.
How to Identify Limestone
Identifying limestone in the field or in a laboratory setting involves a combination of visual inspection, simple chemical tests, and, in some cases, more detailed geological analysis. The following are key methods used to identify limestone:
Visual and Textural Characteristics
- Color: Limestone predominantly exhibits colors ranging from white to gray. However, it can also have hues of yellow, red, or black due to impurities like iron oxide or organic materials.
- Texture: It often has a granular or clastic texture. Fossiliferous limestone may contain visible fossils, and coarser varieties like coquina are composed largely of broken shell fragments.
- Grain Structure: In types like oolitic limestone, small, spherical grains called ooids are visible, while chalk, another form of limestone, is typically fine-grained and smooth.
Chemical Testing
- Acid Reaction Test: A definitive test for limestone is to apply a few drops of dilute hydrochloric acid (or even vinegar in a pinch). If it fizzes and releases carbon dioxide, it’s a strong indication of limestone. This reaction is due to the presence of calcium carbonate.
- Strength of Reaction: The vigor of the acid reaction can also give clues about the purity of the limestone. A stronger fizz suggests a higher concentration of calcium carbonate.
Geological Context
- Surrounding Rock Types: Understanding the geological context of where the rock was found can provide clues. Limestone is often found in layers between other sedimentary rocks.
- Associated Landforms: If the rock is located in an area known for karst features like caves or sinkholes, it’s more likely to be limestone.
Specialized Methods
- Thin Section Analysis: Under a microscope, limestone’s crystalline structure can be examined, revealing details about its mineral composition and formation history.
- Streak Test: Rubbing the rock on a streak plate (unglazed porcelain) to observe the color of its powder can also aid identification. Limestone typically leaves a white streak.
Uses for Limestone

Limestone is useful across various industries and aspects of daily life. Its applications range from construction and architecture to agriculture and beyond, highlighting its importance in human civilization.
Construction and Architecture
Limestone has been a cornerstone in the building industry for centuries, valued for its durability and aesthetic appeal. It is a fundamental material in constructing buildings, facades, and interior decorations. In the realm of construction, its most critical role is as a primary ingredient in cement, essential for creating concrete and mortar. Additionally, crushed limestone is extensively used in landscaping, serving as a pathway material, a base for concrete and asphalt paving, and as an aesthetic element in garden design.
Industrial and Chemical Uses
In the steel manufacturing industry, limestone is indispensable as a flux to remove impurities during the smelting process. Its role extends to the chemical industry where it serves as a raw material for producing various chemicals like slaked lime, quicklime, and hydrated lime, integral in many industrial processes. Furthermore, limestone’s contribution to glass manufacturing is notable, as it enhances the durability and chemical properties of glass products.
Environmental Applications
Limestone plays a crucial role in environmental preservation and management. As agricultural lime, it is used to balance the pH levels of acidic soils, improving soil fertility. Its significance in water treatment is well-established, as it effectively neutralizes acidic waters in both drinking water purification and sewage treatment processes. Moreover, in the effort to control air pollution, limestone is used to remove sulfur dioxide emissions from power plant exhausts, a critical step in flue gas desulfurization processes.
Health and Consumer Goods
The reach of limestone extends into the pharmaceutical and cosmetics industries, where it is used in products like toothpaste, dietary supplements, and certain cosmetics due to its calcium content and mild abrasive properties. In the food industry, it serves as a calcium additive in flour and animal feeds and as an acidity regulator, ensuring the quality and safety of processed foods.
Art and Cultural Heritage
Historically, limestone has been a favored medium for sculptures and architectural carvings, attributed to its workability and aesthetic qualities. It has also been the material of choice for many ancient monuments and buildings, including the Great Pyramids of Giza and numerous medieval churches, a testament to its enduring nature.
Educational and Scientific Significance
In the fields of geology and paleontology, limestone is invaluable. Often containing fossils, it provides crucial insights into Earth’s past environments and life forms, making it an essential material for educational and research purposes.
Facts about Limestone
- Limestone formations can date back to the Precambrian era, providing insights into early Earth conditions.
- Rich in fossils, limestone is key to studying past life forms and environments.
- Composed primarily of calcium carbonate, limestone’s composition varies with impurities like silica, clay, and organic matter.
- Characteristically reacts with acids, fizzing and releasing carbon dioxide, due to its calcium carbonate content.
- The hardness of limestone can significantly vary, affecting its use in different applications.
- Limestone has been used in construction for thousands of years, including in the Egyptian Pyramids and ancient Roman structures.
- Favored by sculptors throughout history for its workability and durability.
- Plays a crucial role in the global carbon cycle as a carbon sink, locking away carbon dioxide.
- Essential in various industries, including steel production, cement manufacturing, and agriculture.
- Responsible for karst landscapes, featuring unique topographical features like sinkholes and caves.
- Found globally, limestone formations such as the Great Barrier Reef and the White Cliffs of Dover are notable for their natural beauty and geological importance.