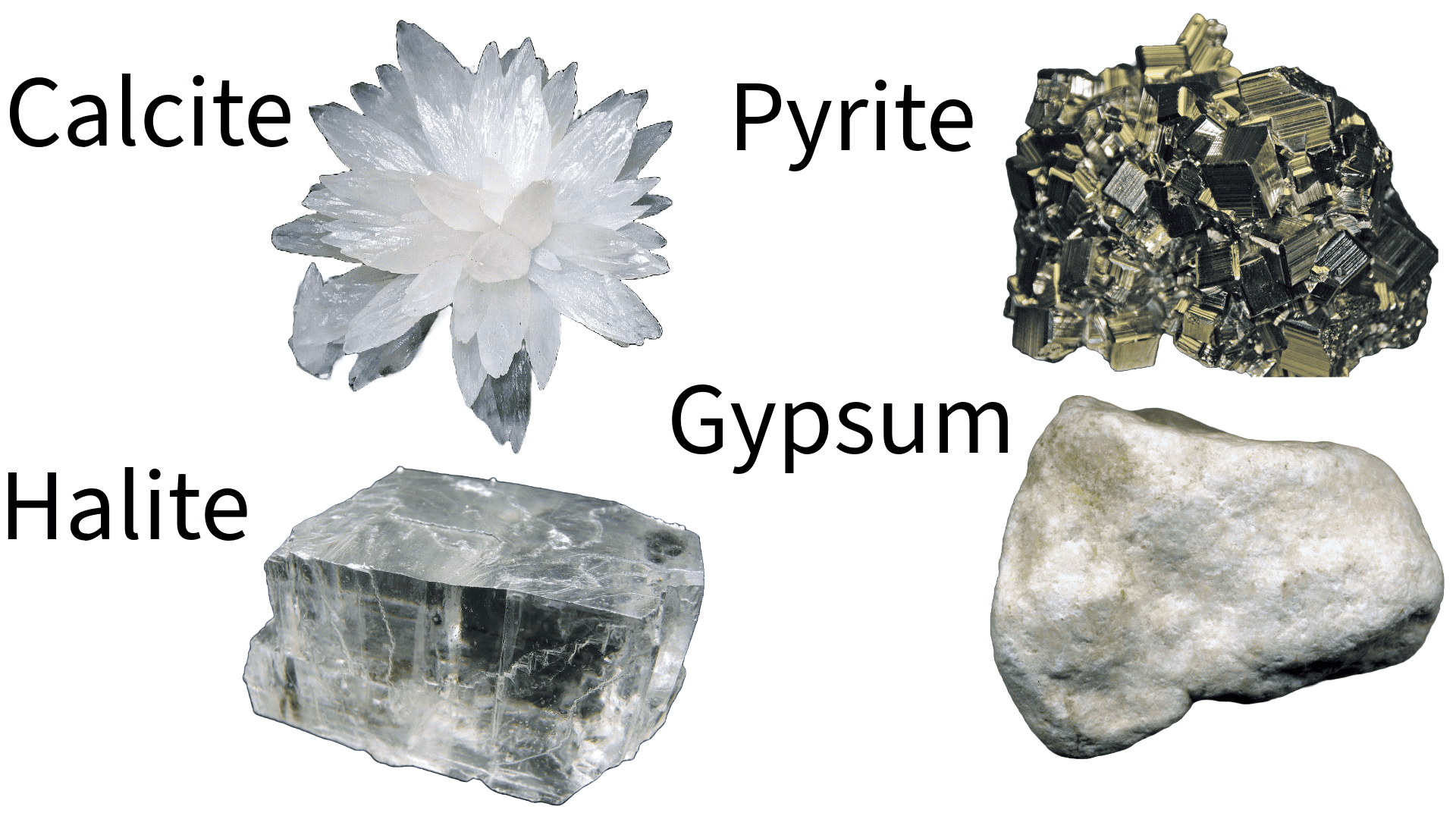
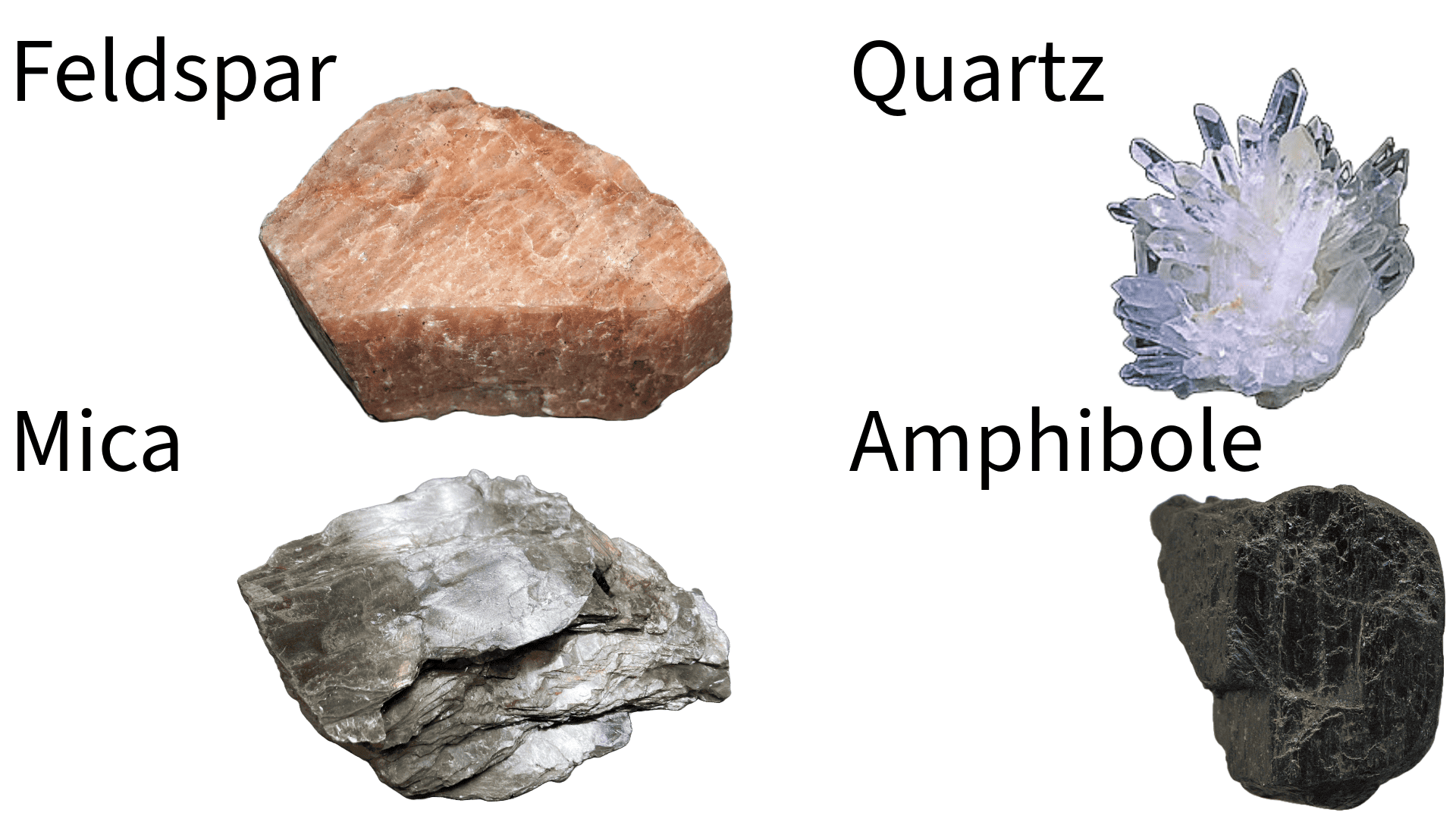
Minerals are naturally occurring, inorganic substances characterized by a definite chemical composition and, typically, a crystalline structure. This structure, formed by the orderly and repeating pattern of atoms, is crucial for determining the physical and chemical properties of the mineral. Except some minerals don’t have it.
Amorphous minerals, in contrast to their crystalline counterparts, lack a well-ordered atomic structure. In fact, “amorphous minerals” is a bit of a misnomer, as minerals cannot be amorphous — but they are mineral-like. Here’s what I mean.
Introduction to Amorphous Minerals
If you’ve ever wondered why nature sometimes creates crystals with near-perfect forms, like the cubic pyrite or the sheet-like mica, it’s all to do with the crystal lattice. Basically, atoms are arranged in the same fashion in a repeating pattern, in all three dimensions. If that lattice happens to be cubical, the crystal can also be cubical (though most crystals are not as neatly arranged).
Crystals are like this because the atoms fall into their lowest stable energy levels. This process is most easily visible in minerals that form from the slow cooling of magma, which creates large, often striking crystals.
But what happens when the minerals cool down too quickly?
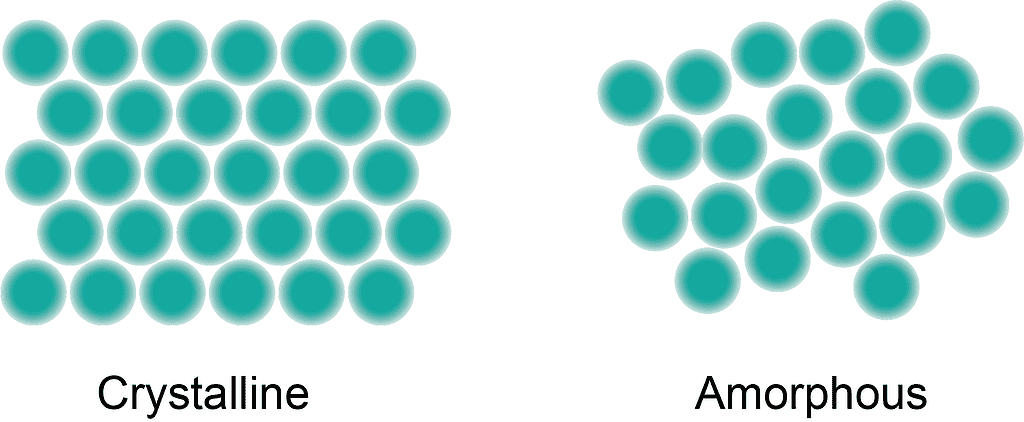
One common way amorphous minerals form is by the rapid cooling of silica-rich volcanic materials. For example, obsidian is an amorphous mineral that results from the rapid cooling of volcanic lava, preventing the formation of a crystalline structure. This chaotic arrangement of molecules is called an “amorphous” structure.
Amorphous minerals don’t technically exist
Minerals, by definition, have to have a crystal structure. They cannot be amorphous. Despite their lack of crystalline structure, however, these exhibit properties that make them of interest to geologists, both scientifically and economically.
So to be technically correct, there are no amorphous minerals. They are amorphous, mineral-like solids.
Examples of amorphous “minerals”

Most elements in this class of amorphous materials revolve around some type of silica.
- Opal
- Composition: Hydrated silica (SiO₂·nH₂O)
- Formation: Forms from the precipitation of silica-rich water in cracks and voids in rocks. The silica is often derived from the dissolution of siliceous minerals and volcanic ash.
- Obsidian
- Composition: Silica-rich volcanic glass
- Formation: Forms when felsic lava extruded from a volcano cools rapidly with minimal crystal growth. It’s essentially a natural glass.
- Geyserite
- Composition: Mostly silica (SiO₂)
- Formation: Deposited around the openings of geysers and hot springs, as the high silica content in the water precipitates upon cooling.
- Fulgurite
- Composition: Various minerals, primarily silica and alumina.
- Formation: Forms when lightning strikes sandy or siliceous ground, fusing the materials together into an amorphous glass.
- Tektites
- Composition: Silica-rich glass
- Formation: Believed to form from the melting and rapid cooling of terrestrial rocks during meteorite impacts. The process creates a glassy, amorphous material scattered over large areas.
- Biogenic Silica
- Composition: Amorphous silica (SiO₂)
- Formation: Produced by organisms such as diatoms and radiolarians in both marine and freshwater environments. After these organisms die, their silica-rich skeletons can accumulate to form deposits.
Why we should care about amorphous “minerals”

So if they’re not technically minerals and there’s not that many of them, why should we even bother with them?
Historically, the focus of mineralogical studies was predominantly on crystalline minerals due to their clear-cut structure and identifiable properties. This bias overshadowed the study of amorphous minerals, whose lack of a regular atomic arrangement challenged traditional mineral identification methods. However, the advent of colloid chemistry and advancements in microscopic and spectroscopic techniques have paved the way for recognizing the importance of amorphous minerals.
Amorphous minerals exhibit unique physical and chemical properties. Their structure, often described as glassy or gel-like, results from the random distribution of atoms or molecules. This lack of order affects their melting points, solubility, and how they interact with light. The presence of water within their structure, forming hydrogels in some cases, further distinguishes amorphous minerals from their crystalline counterparts.
Identifying amorphous minerals involves distinguishing them from crystalline substances without relying on the typical crystallographic methods. Techniques such as X-ray diffraction (XRD), which reveals the absence of a regular pattern, and spectroscopic methods that analyze the chemical bonds and molecular structure, are crucial. Additionally, studying their optical properties, such as refractive indices and the way they scatter light, provides valuable insights into their identification and classification.
Among the amorphous minerals, opal, limonite, and collophane stand out due to their prevalence and applications. Opal, with its play-of-color, is highly valued as a gemstone. Limonite, an iron ore, plays a critical role in the coloration of soils and as a pigment. Collophane, a source of phosphate, is essential for fertilizer production. Each of these minerals exemplifies the diverse formation processes and utility of amorphous minerals in natural and human-made environments.
Colloid chemistry has been instrumental in deepening our understanding of amorphous minerals. It explains the behavior of mineral colloids in aqueous environments, shedding light on their stability, aggregation, and the conditions under which they can transition between amorphous and crystalline forms. This branch of chemistry provides a framework for exploring the nuances of mineral solubility, mobility, and the geochemical cycles affecting amorphous minerals, offering a more comprehensive understanding of their role in the Earth’s system.
Economic importance of amorphous minerals
Amorphous minerals hold significant economic importance due to their unique properties and wide range of applications. Opal, for instance, is highly valued as a gemstone, with its iridescent colors making it desirable for jewelry, fetching high market prices. Obsidian, with its sharp edges and smooth surfaces, has been used historically for tools and ornaments, and today, it’s utilized in decorative items and even some surgical instruments due to its precision cuts.
In environmental contexts, amorphous minerals contribute to soil fertility and water filtration processes. Their ability to adsorb contaminants and facilitate nutrient exchange makes them useful for maintaining the health of natural ecosystems. Moreover, the study of amorphous minerals can provide insights into past climatic conditions and geological events, offering valuable data for environmental science and climate change research.
Silica gel, a form of amorphous silica, is crucial in various industries for its desiccant properties, used extensively in packaging to control humidity and protect goods during shipping. Moreover, the production of industrial glass, ceramics, and refractory materials heavily relies on amorphous minerals, particularly for their melting and molding properties.
Technologically, the unique properties of amorphous minerals, such as their optical characteristics and chemical reactivity, have been harnessed in various industries. From the production of ceramics and glasses to applications in electronics and pharmaceuticals, the versatility of these materials cannot be overstated.
In conclusion, amorphous minerals have emerged as subjects of immense scientific and practical interest. Their diverse properties, environmental significance, and technological applications underscore the importance of expanding our understanding and exploration of these remarkable substances.