Every bit of matter around you is held together by chemical bonds. Simply put, a chemical bond is the force that holds two or more atoms together to form a molecule. Sometimes, chemical bonds are broken, such as during a chemical reaction, only for atoms to bond again to form different molecules. Energy is always released to generate bonds and, likewise, energy is always required to break bonds.
When it comes to the world of chemistry, two types of chemical bonds reign supreme: covalent and ionic bonds. These bonds play a critical role in the chemical reactions that occur all around us, from the food we eat to the air we breathe. But what exactly are covalent and ionic bonds, and what sets them apart? Let’s take a closer look.
How ionic bonds form
Atoms bond together to form compounds because in doing so they attain lower energies than they possess as individual atoms, becoming more stable in the process. By the Law of Conservation of Energy, when a new chemical bond is formed, the chemical reaction releases an amount of energy (usually as heat) almost equal to the difference in the amounts of stored chemical energy between the products and the reactants. This stored chemical energy of the system, or heat content, is known as its enthalpy.
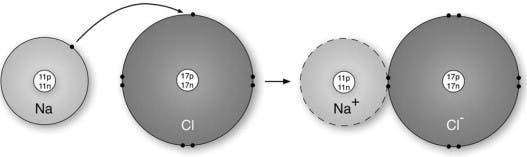
An ionic bond forms when two ions of opposite charges exchange electrons between them, where an ion is an atom that has either lost or gained an electron. Ions that lose one or more electrons have more protons than electrons, which means they have a positive charge. Such ions are called cations (metals). On the other hand, gaining electrons grants the ion a negative charge. Chemists refer to such ions as anions (non-metals).
Ionic compounds are typically neutral. Therefore, ions combine in ways that neutralize their charges.
A textbook example of an ionic compound is sodium chloride, also known as table salt. A single sodium atom has 11 protons and 11 electrons, but only a single electron in its outer shell (or valence shell). Chlorine is made up of 17 protons and 17 electrons, and has 7 electrons in its outer shell. When the two atoms react, sodium (electropositive) loses its valence electron to chlorine (electronegative). Now, in the resulting crystal structure, each sodium ion is surrounded by six chloride ions and each chlorine ion is surrounded by six sodium ions. What’s more, each ion has a complete electron shell that corresponds to the nearest inert gas; neon for a sodium ion, argon for a chloride ion
How covalent bonds form
Covalent bonds form when atoms or ions share electrons such that their outer shells become occupied. Covalent bonds, also called molecular bonds, only form between nonmetal atoms with identical or relatively close electronegativity values, as these elements have a strong tendency to gain or lose electrons, making the sharing of electrons a more favorable option. Electronegativity, denoted by the symbol χ, is a chemical property that describes the tendency of an atom to attract a shared pair of electrons (or electron density) toward itself.
The number of covalent bonds an atom can form is called the valence of the atom. This property represents the electrons of an atom that can participate in the formation of chemical bonds with other atoms. They are the furthest electrons from the nucleus.
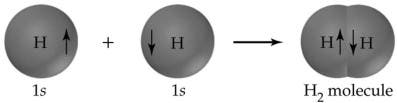
A prime example of a covalent bond is the hydrogen molecule, which forms from two hydrogen atoms, each with one electron in its outer shell. Bond formation releases heat; therefore, it is exothermic. For the hydrogen molecule, the heat released during its formation, also known as the standard enthalpy change (ΔH°), is −435 kJ per mole. The reverse process, breaking the H—H bond, requires 435 kJ per mole, a quantity called the bond strength.
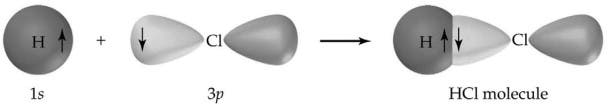
Another classic example of a covalent bond is hydrogen chloride (HCl), which is a hydrogen halide. The chlorine atom has 7 atoms in its outer shell while hydrogen has 1 electron in its outer shell. Both combine perfectly so each atom fills its valence shells, forming a highly stable molecule. Now, the HCl molecule will not react further with other chlorine or hydrogen atoms.
Differences between ionic and covalent bonds
| Property | Ionic Bonds | Covalent Bonds |
|---|---|---|
| Formation | Electrons are transferred from one atom to another | Electrons are shared between atoms |
| Bond Strength | Stronger due to the strong electrostatic forces of attraction between ions | Weaker than ionic bonds |
| Melting and Boiling Points | Higher melting and boiling points due to the strong ionic bonds | Lower melting and boiling points due to the weaker intermolecular forces |
| Solubility | More soluble in polar solvents, such as water | More soluble in nonpolar solvents, such as alcohol or acetone |
| Examples | Table salt (NaCl), Calcium Chloride (CaCl2) | Water (H2O), Carbon Dioxide (CO2) |
- Covalent bonds are much more common in organic chemistry than ionic bonds.
- In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons.
- The reaction components of covalent bonds are electrically neutral, whereas for ionic bonds they are both charged. This explains why sodium chloride (salt) conducts electricity when dissolved — its components are charged.
- Ionic bonds are much stronger than covalent bonds due to the strong electrostatic forces of attraction between ions.
- Covalent bonds are far more common in nature than ionic bonds. Most molecules in living things are covalently bonded, for instance.
- Covalent bonds can form between atoms of the same elements (i.e. H2). However, ionic bonds cannot do this.
- Covalent bonds are formed between two non-metals, whereas ionic bonds are formed between a metal and a non-metal.
- Molecules formed by covalent bonds have low melting and boiling points, whereas those with ionic bonds have high melting and boiling points.
- At room temperature, covalently bonded molecules are in the vast majority of cases liquids or gases, whereas ionic compounds are solid.
- Solubility is another property that sets covalent and ionic compounds apart. Covalent compounds tend to be more soluble in organic solvents, such as alcohol or acetone, while ionic compounds are more soluble in polar solvents, such as water.
Similarities between ionic and covalent bonds
- Both types of bonds lead to the formation of stable chemical compounds.
- It takes exothermic reactions (i.e. that release heat) in order to create ionic and covalent bonds.
- Valence electrons are involved in both bonding processes.
- It doesn’t matter whether a molecule is formed through ionic or covalent bonding as far as its electrical charge is concerned: the result is always electrically neutral.
In conclusion, covalent and ionic bonds are two types of chemical bonds that play a critical role in the world of chemistry. Covalent bonds are formed through the sharing of electrons between atoms, while ionic bonds are formed through the transfer of electrons. While both types of bonds are essential, they have distinct differences in their properties, including strength, melting and boiling points, and solubility. Understanding these differences is crucial for understanding the chemical reactions that occur all around us.