
When we think of atoms, we often picture tiny spheres with electrons whizzing around a nucleus. But did you know that not all atoms are created equal? While they may look the same, some atoms have different numbers of neutrons in their nucleus, giving them different properties. These variants of an element are called isotopes.
Isotopes have unique properties, and these properties make them useful in a wide variety of fields, such as medicine, geology, and even archaeology.
What are isotopes?
Isotopes are atom families that have the same number of protons, but different numbers of neutrons. The term is drawn from ancient Greek words isos and topos, meaning ‘equal place’, to signify that they belong to the same elements on the periodic table.
To understand isotopes, we first need to understand the structure of an atom. At the center of an atom is its nucleus, which contains protons and neutrons. But not all atoms of the same element have the same number of neutrons. Carbon, for example, can have six, seven, or eight neutrons in its nucleus. Each of these variants is a different isotope of carbon, known as carbon-12, carbon-13, and carbon-14, respectively.
Isotopes are named based on the total number of protons and neutrons in their nucleus. Carbon-12, for example, has six protons and six neutrons, while carbon-13 has six protons and seven neutrons.
To get a bit more technical, the number of protons within an atom’s nucleus is its ‘atomic number’ (aka the ‘proton number‘, usually notated ‘Z‘). What’s important right now is to keep in mind that these atomic numbers identify individual elements. The atomic number is roughly equivalent to an element’s numeric place in the periodic table, and in broad lines dictates how an element tends to behave. All isotopes of an element have the same atomic number. What they differ in is their ‘mass number‘ (usually abbreviated ‘A‘), which denotes the total number of protons and neutrons in an atom’s core.
In other words, isotopes are atoms of the same element — but some just weigh more.
For example, two isotopes of Uranium, U-235 and U-238, have the same atomic number (92), but mass numbers of 235 and 238, respectively. You can have two isotopes of the same mass, like C-14 and N-14, that aren’t the same element at all, with atomic numbers 6 and 7, respectively. To find out how many neutrons an isotope harbors, subtract its atomic number from its mass number.
Image via Pixabay.
Two kinds of isotopes
Isotopes can be divided into two broad categories: stable and unstable. The two main facts that determine the stability of nuclei are the ratio of protons to neutrons and the sum of protons and neutrons.
Stable isotopes do not emit radiation whereas unstable isotopes emit radiation. Some elements have no stable isotopes, which means that any atom of that element is radioactive, such as in the case of uranium. For some other elements, only certain isotopes are radioactive.
For instance, Carbon-12, with six protons and six neutrons, is a stable nucleus, which means it does not spontaneously emit radioactivity. However, Carbon-14, with its six protons and eight neutrons, is unstable and naturally radioactive. Radioactive decay can happen in several ways, including emitting particles or energy, or splitting into smaller nuclei.
Taken together, the 81 stable elements known to us can boast some 275 stable isotopes. There are over 800 more radioactive (unstable) isotopes out there — some are natural, and some we’ve created in the lab.
Where do isotopes come from?
Long story short, isotopes are simply atoms with more neutrons — they were either formed that way, enriched with neutrons sometime during their life, or originate from nuclear processes that alter atomic nuclei. So, they form like all other atoms.
Lighter isotopes likely came together a bit after the Big Bang, while heavier ones were synthesized in the cores of stars. Isotopes can also form following the interaction between cosmic rays and energetic nuclei in the top layers of the atmosphere.
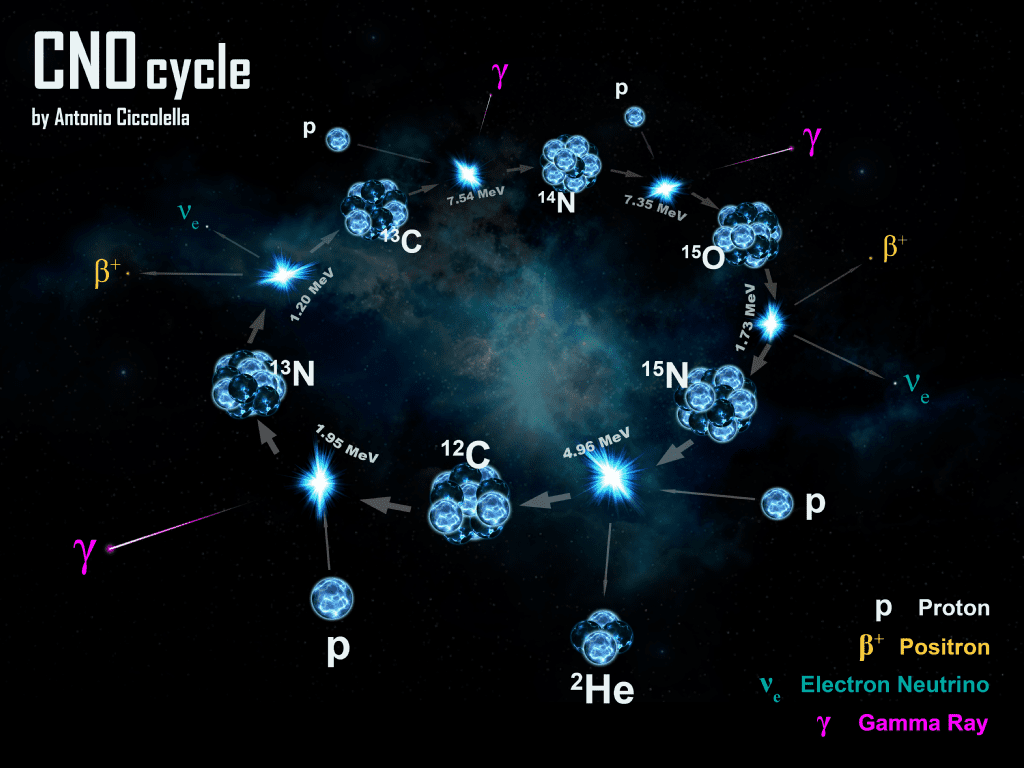
Image credits Antonio Ciccolella / Wikimedia.
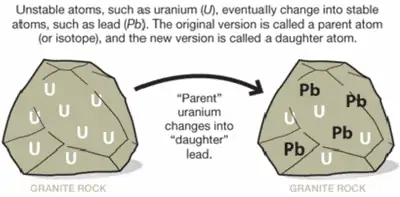
Isotopes can also be formed from other atoms or isotopes that have undergone changes over time. One example of such a process is radioactive decay: basically, unstable isotopes tend to shift towards a stable configuration over time. This can cause one unstable isotope to change into a stable one of the same element, or into isotopes of other elements with similar nucleic structures. U-238, for example, decays into Th-234.
This process occurs when there are too many protons compared to neutrons in a nucleus (or vice-versa). In the example above, the uranium atom is the parent isotope, while the thorium atom is the daughter isotope. During this process, the nucleus emits radiation in the form of an electron and an antineutrino.
What are isotopes good for? Quite a lot of things actually
One of the prime uses for isotopes is dating (like carbon dating). One particular trait of unstable isotopes is that they decay into stable ones — but they always do so at the exact same rate. For example, C-14’s half-life (the amount of time needed for half of all isotopes in a sample to decay) is 5,730 years.
C-14 is formed in the atmosphere, and while an organism is alive, it ingests about one C-14 atom for every trillion stable C-12 isotopes through the food it eats. This keeps the C-12 to C-14 ratio roughly stable while it is alive. Once it dies, intake of C-14 stops — so by looking at how many C-14 atoms a sample has, we can calculate how far down C-14’s half-life it’s gone, meaning we can calculate its age.
At least, in theory. All our use of fossil fuels is pumping more C-14 isotopes into the atmosphere than normal, and it’s starting to mess up the accuracy of carbon dating. To see how many C-14 atoms something has, we use accelerator mass spectrometry — a method that separates isotopes via mass.
Similarly, uranium-lead dating works by measuring the ratio of uranium-238 to lead-206 in a sample. Because uranium-238 decays into lead-206 at a known rate, the ratio of the two isotopes can be used to calculate the age of the sample. The half-life of uranium-238 is so large that we can use this method to determine the age of some of the oldest rocks, some of which can be as old as 4.5 billion years — that’s the age of Earth.
Isotopes also have important applications in medicine. One example is cancer treatment, where radioactive isotopes can be used to target and destroy cancer cells.
One such isotope is iodine-131, which is taken up by the thyroid gland. By attaching iodine-131 to a molecule that is preferentially taken up by cancer cells, doctors can deliver a high dose of radiation directly to the tumor, sparing healthy tissue. Other isotopes, like technetium-99m, are used in medical imaging. This isotope emits gamma rays, which can be detected by a special camera and used to create detailed images of the body’s internal organs and tissues. This allows doctors to diagnose a wide range of medical conditions, from heart disease to cancer, with a non-invasive procedure.
While unstable isotopes like carbon-14 are commonly used in archaeology for dating, stable isotopes also have an important role to play. By analyzing the ratios of stable isotopes like oxygen-18 and carbon-13 in materials like bone and teeth, scientists can learn about the diet and migration patterns of ancient people.
For example, by analyzing the oxygen-18 content of teeth, scientists can determine where an individual grew up, as the oxygen-18 content of drinking water varies depending on location. Similarly, by analyzing the carbon-13 content of bones, scientists can determine what types of foods an individual ate, as different foods have different carbon-13 signatures.
Finally, we sometimes create ‘enriched’ materials, such as enriched uranium, to be used in nuclear reactors. This process basically involves us weeding through naturally-occurring uranium atoms via various methods for heavier isotopes, then separating those. The metal that we’ve already removed the heavier isotopes from (which are more unstable and thus more radioactive than ‘regular’ uranium) is known as ‘depleted uranium’.
Do isotopes of the same element behave differently?
For the most part, no. Generally speaking, there’s little to no difference in how various isotopes of the same element behave. This is partly a function of how we decide what each element ‘is’: roughly three-quarters of naturally-occurring elements are a mixture of isotopes. The average mass of a bunch of these isotopes put together is how we determine those elements’ standard atomic weights.
But, chiefly, it comes down to the point we’ve made previously: without differences in their electron shell, isotopes simply lack the means to change their chemical behavior. Which is just peachy for us. Taken together, the 81 stable elements known to us can boast some 275 stable isotopes. There are over 800 more radioactive (unstable) isotopes out there — some natural, and some we’ve created in the lab. Imagine the headache it would cause if they all behaved in a different way. Carbon itself has 3 stable isotopes — would we even exist today if each had its own quirks?
One element whose isotopes do differ meaningfully, however, is the runt of the periodic table: hydrogen. This exception is based on the atom’s particular nature. Hydrogen is the simplest chemical element, one proton orbited by one electron. Therefore, one extra neutron in the core can significantly alter the atom’s properties.
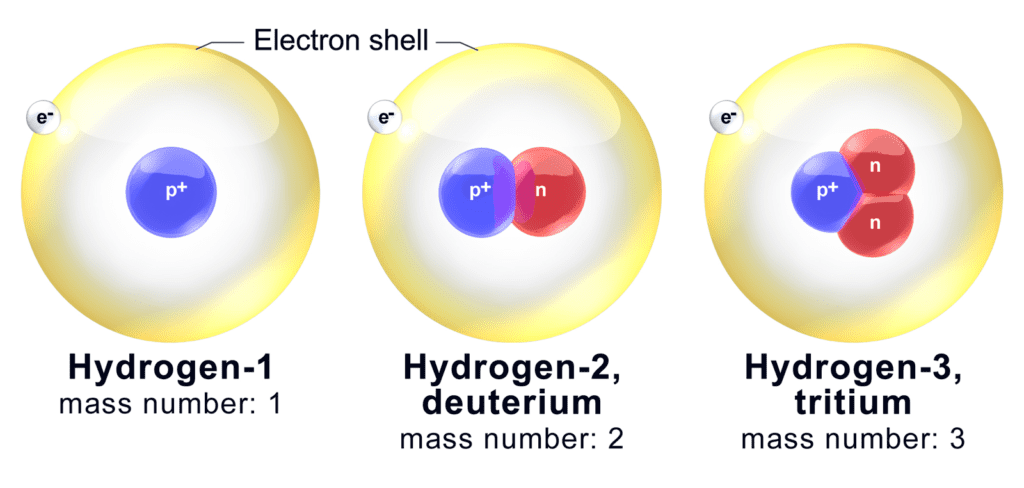
Image credits BruceBlaus / Wikimedia.
For example, two of hydrogen’s natural isotopes, H-2 and H-3, have 1 and 2 neutrons respectively. Carbon (Z=6) has 2 stable isotopes: C-12 and C-13, with 6 and 7 neutrons respectively. In relative terms, there isn’t a huge difference in the neutrons’ share in their cores: they represent 50%, and 66.6% of the atoms’ weight in H-2, H-3, and 50% and 54-ish% of the total mass in C-12 and C-13. In absolute terms, though, the difference is immense: one neutron will double the mass of a hydrogen atom — two neutrons will triple it. For comparison, a single neutron is just 16.6% of a carbon atom’s mass.
While isotopes are highly similar chemically, they do differ physically. All that weight can alter how isotopes of light elements, hydrogen especially, behave. One example of such differences is the kinetic isotope effect — basically, heavier isotopes of the same element tend to be more sluggish during chemical reactions than lighter isotopes. For heavier elements, this effect is negligible.
Another quirky property of isotopes is that they tend to behave differently when exposed to infrared range than the ‘default’ elemental atoms. So, molecules that contain isotopes will look different from the same molecule sans isotopes when seen through an infrared camera.
Conclusion
By understanding the properties and applications of isotopes, we can gain a deeper appreciation for the incredible complexity and interconnectedness of the natural world. Whether we’re exploring the origins of the universe or developing new treatments for disease, isotopes are sure to play a key role in shaping the future of science and technology.






