Have you ever noticed a fuzzy film growing on a rock in a stream or a slime coating on the inside of a water bottle? These are examples of biofilms, complex communities of microorganisms that live together in a self-produced matrix of extracellular polymeric substances (EPS).
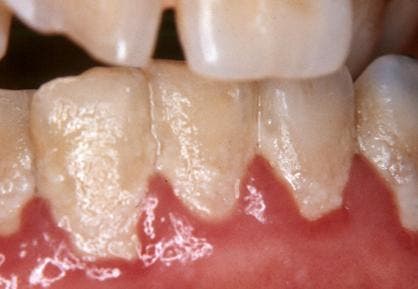
The microorganisms that form biofilms include bacteria, fungi, and protists. Perhaps the most common biofilm familiar to most is dental plaque — that sticky, colorless film of bacteria and sugars that constantly forms on our teeth. That slime that sometimes forms on the surface of water, particularly in ponds, is also biofilm.
Actually, we’ve found biofilms almost anywhere; on minerals, metal surfaces, and inside our guts. In fact, biofilms have been around for at least 3.3 billion years. However, it’s in wet and moist environments that you’ll the most biofilms. They love wetness.
A vast number of pathogens are grouped as biofilms. Like humans, they’ve learned this configuration enhances their survival rate as there’s strength in numbers and they are better able to fight the cells sent by the immune system bent upon destroying them.
How do biofilms form?
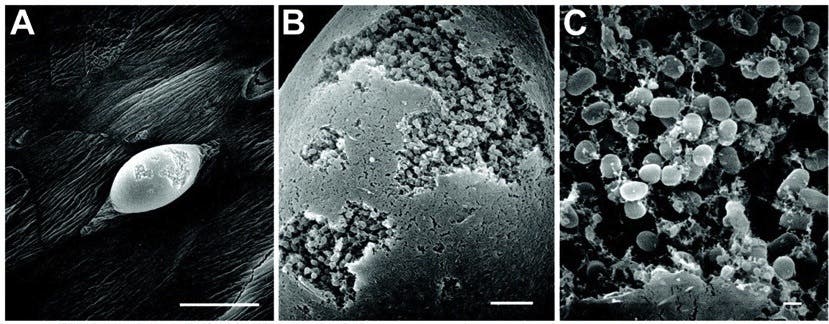
The slimy films start forming when initially free-floating bacteria adhere to surfaces in aqueous environments and start ‘laying their roots’. To stay sticky, the bacteria excrete a glue-like substance that’s effective at anchoring them to all kinds of materials, from plastics to soil to medical implants such as pacemakers. This glue is known as an extracellular polymeric substance (EPS) and is comprised of sugars, proteins, and nucleic acids like DNA.
In time, layers upon layers of EPS are added. After a period of growth, a complex 3D structure emerges which is packed with water channels on the inside that facilitate the exchange of nutrients and waste products.
What’s particularly fascinating is how the bacteria communicate within these biofilm communities. Unlike their free-living counterparts, the microorganisms in biofilms work together in a synchronized fashion to protect and nourish their community. Microbes can instruct each other where to position themselves through quorum sensing. Basically, this phenomenon allows a single-celled bacteria to sense how many other bacteria are there in its close proximity. If the bacteria senses there’s a dense population surrounding it, it will be inclined to join them.
This cooperative behavior allows biofilms to thrive in environments that would otherwise be hostile to individual microorganisms. Remember, strength in numbers.
“Disease-causing bacteria talk to each other with a chemical vocabulary,” says Doug Hibbins of Princeton University.
“Forming a biofilm is one of the crucial steps in cholera’s progression,” said Dr. Bonnie Bassler, a microbiologist also at Princeton. “They [bacteria] cover themselves in a sort of goop that’s a shield against antibiotics, allowing them to grow rapidly. When they sense there are enough of them, they try to leave the body.”
Sometimes clumps of biofilm can break away from the main mass and establish themselves on a new surface. These new pioneers will continue to extend their slimy film until they form a new, bigger colony, starting the cycle over again.
How big can a biofilm get?
Most biofilms are very thin — just a few cell layers thick. That’s too thin to see with the naked eye. In fact, your kitchen counter almost certainly has a biofilm layer on it. You just can’t see it. Some biofilms, however, can grow many inches thick and are obviously noticeable. You’ll find these thick slime molds growing as algae on rocks in a streambed, for instance.
The thickness of biofilms depends on several environmental factors. Some organisms can produce large amounts of EPS and hence grow a thicker biofilm. Water flow is also an important factor or, to be more precise, shear stress. If a biofilm forms in a creek where there’s a high flow of water, it ought to be fairly thin. Biofilms formed in slow-flowing or stationary water, like a pond, can grow quite thick.
Why do biofilms form?
Bacteria band together because as a community they enhance their odds of survival, but what threats do they face and how does living in a slime mold protect them? Some of the stressors individual bacteria face are the lack of water, high or low pH, or the presence of ‘toxic’ substances, i.e. antibiotics or antimicrobials.
The EPS layers act as the first line of defense against these threats. It can prevent dehydration or shield the bacteria against UV radiation. When antimicrobials, bleach, or even metals come in direct contact with the EPS, they become bounded and neutralized by the sticky EPS.
Antibiotics can certainly destroy biofilm but not always because biofilms employ another line of defense. For instance, despite some antibiotic substances might penetrate the EPS layer, they can be met by dormant bacteria, which shield active bacteria present a layer underneath. Because these dormant bacteria lack cellular activity, the antibiotics don’t work their magic because there’s nothing to disrupt.
Another line of defense against antibiotics is represented by the ‘persisters’, a special kind of bacteria that do not divide. These bacteria produce substances that block the targets of many antibiotics, according to a 2010 paper. Compared to free-floating bacteria, those growing as a biofilm can be up to 1,500 times more resistant to antibiotics
Finally, living inside a community, often made of different bacterial species, means its members can reap the benefits that come with having a multi-skilled network. For instance, some biofilms are made of both autotrophic and heterotrophic microorganisms. The autotrophs produce their own food using photosynthesis and available organic material while heterotrophs don’t make their own food and require external sources of carbon. As such, in these biofilms, the microorganisms will often cross-feed. It’s a sort of division of labor.
Biofilms, humans, and disease
Biofilms seem to be able to form and cling to just about any external surface as long as it’s wet. This may naturally beg the question — does that mean they can form inside the human body as well? It certainly is wet enough and, indeed, we find that the answer is ‘yes’. According to the National Institutes of Health, more than 65% of all microbial infections are caused by biofilms. The vast majority of these infections are common like urinary tract infections, catheter infections, dental plaque formation, and so on.
However, biofilms can be involved in a range of nasty diseases and medical problems. One example is kidney stones which are caused by biofilms. Some 15 to 20 percent of kidney stones form as a result of urinary tract infections, produced by the interplay between infecting bacteria and mineral substances from the urine.
Then there’s endocarditis, a disease that involves inflammation of the inner layers of the heart. Endocarditis seems to be triggered by a complex biofilm made from bacterial and host components located on a cardiac valve. This type of biofilm is known as a vegetation. The vegetation can disrupt valve function, produce a near-continuous infection of the bloodstream, and can block blood circulation through a process known as embolization.
Pathogenic biofilms also plague prostheses and various medical implants like artificial joints and heart valves or pacemakers. This first came to the medical community’s attention in the 1980s when bacterial biofilms were found on intravenous catheters and pacemakers.
“When people think of infection, they may think of fever or pus coming out of a wound,” explains Dr. Patel from the Mayo Clinic. “However, this is not the case with prosthetic joint infection. Patients will often experience pain, but not other symptoms usually associated with infection. Often what happens is that the bacteria that cause infection on prosthetic joints are the same as bacteria that live harmlessly on our skin. However, on a prosthetic joint they can stick, grow and cause problems over the long term. Many of these bacteria would not infect the joint were it not for the prosthesis.”
Biofilms have been poorly understudied until recently but evidence suggests they’re involved in many human diseases, including debilitating chronic infections. According to Dr. Trevor Marshall, a biomedical researcher at Murdoch University, Australia, some large microbiota of chronic biofilms, such as L-shaped bacteria, can evade the immune system because a long time ago they evolved the ability to reside inside macrophages. Ironically, these are the very white blood cells of the immune system which are supposed to kill the invading pathogens. Marshall also says that biofilm infections occur with great ease in immunocompromised hosts.
Targeting biofilm infections
Research carried out over the past three decades suggests that biofilms are either extremely difficult or impossible to eradicate from the human body. What’s certain is that administering antibiotics in a standard manner (high dose and consistently over many days) does not work effectively.
After high doses of antibiotics are administered, it may seem the biofilm infection has disappeared. However, it will often reappear because the biofilm was not destroyed, only weakened. It seems that while antibiotics can penetrate the biofilm matrix and kill bacteria, a number of cells called ‘persisters’ are left behind. These are able to survive the onslaught of antibiotics and gradually allow the biofilm to form again.
Dr. Kim Lewis of Tulane University, however, says that it is possible to destroy some biofilms. His treatment involves using pulsed, low-dose antibiotics to break up the biofilm. For instance, research suggests this technique is effective at destroying P. aeruginosa biofilm bacteria in a manner that is indistinguishable when the same antibiotic concentrations are administered to single planktonic cells.
When the low, pulsed dosing of antibiotics is applied, the first application eradicates the bulk of the biofilm cells, leaving the persisters behind. Because the antibiotics are stopped, the survival of the persisters is not enhanced. Lewis believes this causes the cells to lose their shape and biochemical properties, making them unable to restart the biofilm formation process. A second application of the antibiotic after a certain time should then completely eliminate the persister cells. However, the efficacy of this method depends on the ability to manipulate the antibiotic concentration. Furthermore, not all biofilms can be broken down this way.
Recently, researchers have been exploring new strategies to fight biofilm infections, including using bacteriophages, or virus-like particles, to target specific types of bacteria within a biofilm. Another approach involves using natural products such as honey and ginger, which have been shown to have antimicrobial properties against biofilm-forming bacteria.
Useful biofilms
Biofilms can cause serious medical conditions and, as we’ve seen, they can be very difficult to get rid of. But there are instances when biofilms can be useful, for bioremediation purposes. Biofilms are used, for instance, in treating wastewater or water contaminated with heavy metals or radioactive substances. Another practical use for biofilms is in microbial fuel cells. In such fuel cells, microbes that live on the surface of an electrode break down nutrients and transfer electrons through a circuit, providing electricity. Microbial fuel cells can be very useful if you need to remotely generate power for sensors in wastewater or landfills.
Biofilms are right now the subject of intense research. Biofilms cause billions in damage every year due to disease, equipment damage, energy loss or contaminations, so finding ways to get rid of them is a priority. The resilience of biofilms is a big challenge and requires contributions from different fields of science such as biochemistry, engineering, mathematics, and microbiology.