Researchers from the University of Cambridge look to plants for a new energy revolution.
Image credits Luis Romero / Flickr.
Looking for new and more efficient ways of harvesting solar energy, a team of researchers from St John’s College has turned plants to the job. The team has successfully split water molecules into hydrogen and oxygen by altering and improving on natural photosynthetic processes. Photosynthesis is the process plants use to convert sunlight into energy.
Lettuce make fuel
Photosynthesis is arguably the most important process for life on Earth. The process — which uses energy in sunlight to break down water and carbon dioxide — provides the energy and building blocks that plants need to grow. In turn, plants act as primary producers: they form the first link of virtually every trophic network on the planet, essentially feeding the rest of the planet. Moreover, photosynthesis is the source of nearly all the oxygen in the atmosphere today. In its absence, oxygen (a very reactive gas) would bind with chemical compounds or would be used up in biological respiration pretty quickly, and we’d all choke to death. Which would be sad.
Not content to let the process drive just our biology, the team — led by St John’s College PhD student Katarzyna Sokół — worked on turning it into a power source.
Hydrogen has long been considered a viable — and powerful — alternative to fossil fuels. In fact, the first internal combustion engine ever built used a mixture of hydrogen and oxygen, not fossil fuels, to generate energy. It was designed by Francois Isaac de Rivaz, a Swiss inventor, all the way back in 1806. However, it never really caught on, as we didn’t know of any fast and cheap way of mass-producing the gas.
Artificial photosynthesis has yet to reach a point where it can supply enough hydrogen for wide-scale use, mostly because it relies on the use of catalysts, which are often expensive and toxic. On the other hand:
“Natural photosynthesis is not efficient because it has evolved merely to survive so it makes the bare minimum amount of energy needed — around 1-2 per cent of what it could potentially convert and store,” says Katarzyna Sokół, who is also the paper’s first author.
This means that neither can support an industrial-level economy based on hydrogen.
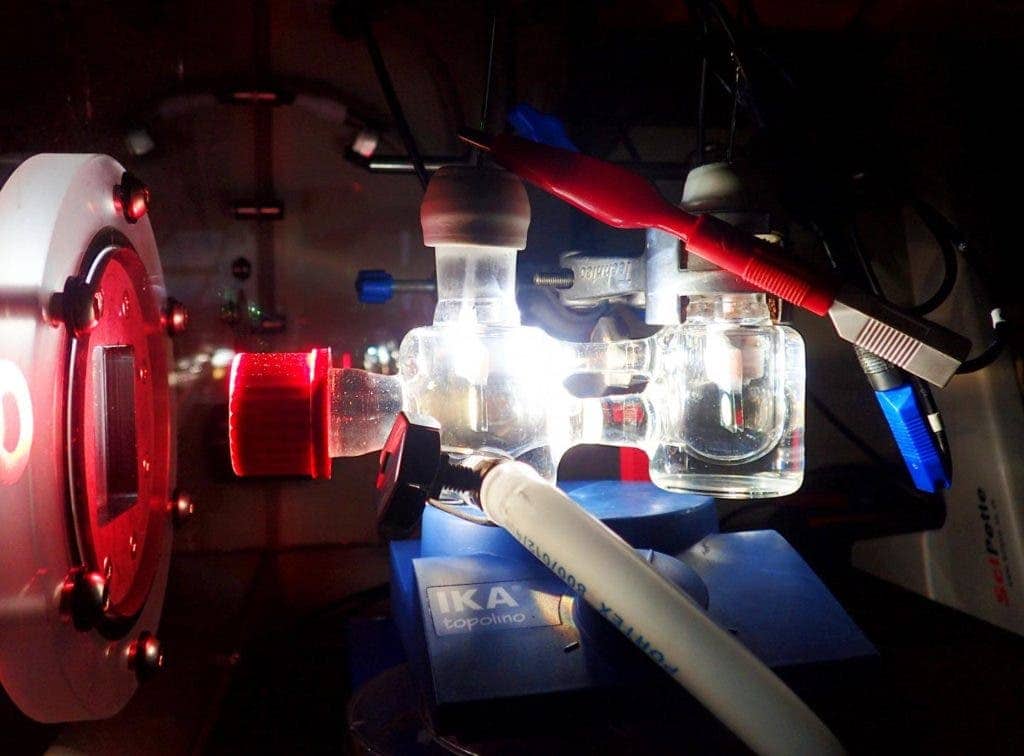
Image credits Katarzyna Sokół.
The team’s new paper details their efforts to change this state of affairs. Using a combination of biological components and manmade technologies, they managed to convert water into hydrogen and oxygen at high efficiency using only sunlight — a process known as semi-artificial photosynthesis. As part of their research, the team had to remove genetic limitations on photosynthesis that had been imposed millennia ago.
“Hydrogenase is an enzyme present in algae that is capable of reducing protons into hydrogen. During evolution this process has been deactivated because it wasn’t necessary for survival,” Sokół explains, “but we successfully managed to bypass the inactivity to achieve the reaction we wanted — splitting water into hydrogen and oxygen.”
The team is the first to successfully create semi-artificial photosynthesis driven solely by sunlight. Their method was over 80% more efficient than natural photosynthesis.
The groundwork they laid down in integrating organic and inorganic materials into semi-artificial devices also provides new avenues of research into other systems for solar energy capture, they add.
“It’s exciting that we can selectively choose the processes we want, and achieve the reaction we want which is inaccessible in nature,” Sokół explains.
“This could be a great platform for developing solar technologies. The approach could be used to couple other reactions together to see what can be done, learn from these reactions and then build synthetic, more robust pieces of solar energy technology.”
The paper has been published in the journal Nature.


