The chemical reactions used to make methanol from carbon dioxide rely on a catalyst to speed up the conversion, and scientists identified a new material that could fill this role. With its unique structure, this catalyst can capture and convert carbon dioxide in a way that ultimately saves energy.
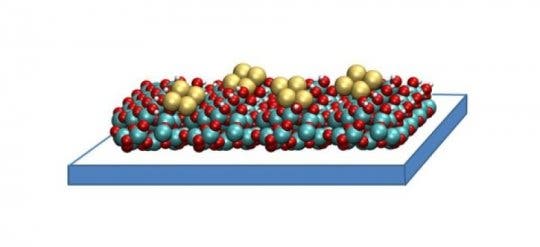
Credit: Image courtesy Larry Curtiss, Argonne National Laboratory
We have covered carbon capture before, so you should be familiar with the underlying idea – capture CO2 and convert it into a easily storable, inert substance. Recent advances gave us new ways to turn this green house gas into useful substances, such as building materials, or use it to produce light or even fuel. One of the products that can be obtained this way, and then be used as fuel is methanol, a pretty simple carbon, oxygen and hydrogen molecule that my chemistry teacher loved to quiz me about so i hate.
Producing methanol this way first requires an efficient method of fishing carbon compounds out of the atmosphere, a process which up to now involved highly-pressurizing gasses over a chemical capture compound made up of copper, aluminium oxide and zinc oxide. A team of researchers working out of the U.S. Department of Energy’s (DOE) Argonne National Laboratory has developed a new compound that they hope will revolutionize the process, as with its unique structure, this catalyst can capture and convert carbon dioxide in a way that ultimately saves energy.
Image via biologycorner
The compound is called copper tetramer. It’s made up of small clusters of four copper atoms each, spread on a thin film of aluminium oxide. These catalysts work by binding carbon dioxide molecules, and orienting them in a way that facilitates chemical reactions. The structure of the copper tetramer is more efficient as most of it’s binding sites are open, so that it can attach more strongly to CO2 and accelerate the conversion. As it stands, a number of the binding sites in the now-used capture compound are occupied merely in holding the substance together, which limits how many atoms can catch and hold carbon dioxide.
“With our catalyst, there is no inside,” said Stefan Vajda, senior chemist at Argonne and the Institute for Molecular Engineering and co-author on the paper. “All four copper atoms are participating because with only a few of them in the cluster, they are all exposed and able to bind.”
This is why the current method calls for high-pressure environments in which the capture to take place, so that stronger bonds form between the CO2 molecules and the bonding compound. But compressing gas into a high-pressure mixture takes a lot of energy. The benefit of enhanced binding is that the new catalyst requires lower pressure and less energy to produce the same amount of methanol.
Carbon dioxide emissions are an ongoing environmental problem, and according to the authors, it’s important that research identifies optimal ways to deal with the waste.
“We’re interested in finding new catalytic reactions that will be more efficient than the current catalysts, especially in terms of saving energy,” said Larry Curtiss, an Argonne Distinguished Fellow who co-authored this paper.
Copper tetramers could allow us to capture and convert carbon dioxide on a larger scale — reducing an environmental threat and creating a useful product like methanol that can be transported and burned for fuel. Of course the catalyst still has a long journey ahead from the lab to industry.
Some of the kinks that still have to be ironed out are the compound’s instability and finding an efficient way to manufacture mass quantities of the substance. There is a chance that the tetramers may decompose in an industrial setting, so ensuring long-term durability is a critical step for future research, Curtiss said. And while only nanograms of the material were required for lab studies, a much higher quantity would be needed for industrial purposes.
Meanwhile, the researchers are interested in searching for other catalysts that might even outperform their copper tetramer.
These catalysts can be varied in size, composition and support material, which results in a list of more than 2,000 potential combinations, Vajda said.
But the scientists don’t have to run thousands of different experiments, said Peter Zapol, an Argonne physicist and co-author of this paper. Instead, they will use advanced calculations to make predictions, and then test the catalysts that seem most promising.
“We haven’t yet found a catalyst better than the copper tetramer, but we hope to,” Vajda said. “With global warming becoming a bigger burden, it’s pressing that we keep trying to turn carbon dioxide emissions back into something useful.”
For this research, the team used the Center for Nanoscale Materials as well as beamline 12-ID-C of the Advanced Photon Source, both DOE Office of Science User Facilities.
Curtiss said the Advanced Photon Source allowed the scientists to observe ultralow loadings of their small clusters, down to a few nanograms, which was a critical piece of this investigation.


