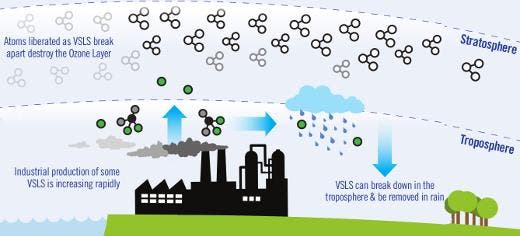
After scientists discovered a huge hole in the ozone layer above the Antarctic, an emergency UN panel banned the use of chlorofluorocarbons (CFCs) in 1987. These build up in the atmosphere, react with the triple oxygen molecule and break it down. Since then, ozone has thankfully replenished, thought it might take decades before it reverts to pre-1980 levels. Progress is slow because there are still some plants through out the world who illegally use CFCs (the stuff that used to go into refrigerants or deodorants), but also because there are other ozone-depleting chemicals out there – some recognized, others new and extremely dangerous. One class of chemicals that has been allowed in the industry since the Montreal Protocol, despite the danger it posses to ozone, is made up of so-called ‘very short-lived substances’ (VSLS) which breakup in under six months. A new study, however, found that these have dramatically increased over the past couple of years and despite their short reaction times, these could prove to be extremely dangerous.
Short lived, but dangerous

At ground level, ozone or smog is a poisonous chemical often expelled by the exhaust of vehicles. High up in the stratosphere, ozone builds up at altitudes between 10 and 50 where it acts as a shield, filtering harmful ultraviolet rays which can cause cancer. Ozone holes occur naturally because of cooling, but man-made chemicals greatly accelerate their formation. Currently, the ozone hole above Antarctica is the size of North America.
Though CFCs have been banned, there are other chemicals that contribute to the Antarctic ozone hole. Ironically, one chemical called dichloromethane that has been officially deemed as a replacement for CFCs following the Montreal Protocol can found in a report authored by researchers at Leeds University which highlights VLSL that deplete the stratospheric shield. These chemicals usually break down in less than six months, but simulations show VSLS account for a significant portion of ozone loss in the stratosphere.
“In the Antarctic region, where the ozone hole forms each year and where ozone decreases are the most dramatic, we estimate that VSLS account for about 12.5 per cent of the total ozone loss.
“Globally averaged, the ozone loss due to VSLS in the lower stratosphere could be as much as 25 per cent, though it is much smaller at higher altitude,” Ryan Hossaini of the University of Leeds (U.K) and lead author of the study,
It’s important to consider than over 90% of VSLS occur from natural processes, like the bromine compounds produced by seaweed and the ocean’s phytoplankton. About 10% are man-made chlorine compounds. At first glace, dichloromethane – one of the most abundant man-made VSLS that we know of – doesn’t seem to be that important. The computer models suggest it reduces the ozone layer by less than one per cent, the researchers note, but atmospheric concentration of dichloromethane has increased dramatically in recent years. In some places, the concentration has doubled since late 1990s levels, which makes it a genuine concern. The findings appeared in Nature Geoscience.
“The increases observed for dichloromethane are striking and unexpected; concentrations had been decreasing slowly in the late 1990s, but since then have increased by about a factor of two at sites throughout the globe,” said study co-author Stephen Montzka of the National Oceanic and Atmospheric Administration.
Scientists aren’t exactly sure what’s causing the growth of dichloromethane.
“It could be partly due to the fact that dichloromethane is used in the manufacturing process of some HFCs, the ozone-friendly gases which were developed to replace CFCs,” Hossaini said.


