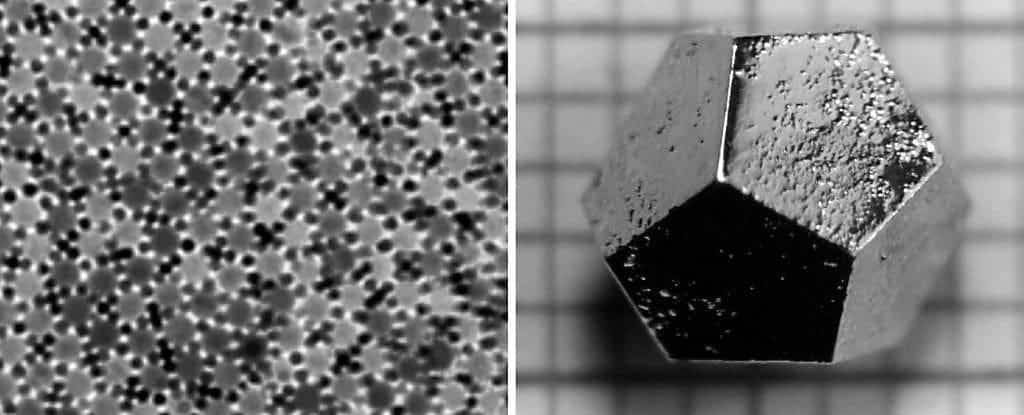
Scientists from Germany have developed by accident a peculiar new substance consisting of 12-sided, non-repeating atomic units. Typically this weird structure is called a quasicrystal, a chemical structure thought impossible a few decades ago. Pioneering work on this subject landed Professor Daniel Shechtman the Nobel Prize for Chemistry in 2011.
Klaus Meinel, Stefan Forster and Wolf Widdra, scientists at Germany’s Martin Luther University, were making trials with an interface made out of two materials under various conditions to engineer properties not found in nature (meta-material). Totally by accident, the team ended up replicating the conditions necessary for the formation of a quasicrystal – crystals that break all the rules of being a crystal at all. Quasicrystals are structural forms that are ordered but not periodic. They form patterns that fill all the space though they lack translational symmetry.
Most crystals are composed of a three-dimensional arrangement of atoms that repeat in an orderly pattern. Depending on their chemical composition, they have different symmetries. For example, atoms arranged in repeating cubes have fourfold symmetry. Atoms arranged as equilateral triangles have threefold symmetries. But quasicrystals behave differently than other crystals. They have an orderly pattern that includes pentagons, fivefold shapes, but unlike other crystals, the pattern never repeats itself exactly.
A crystal that shouldn’t exist
Neither repetitive, nor disorganized in structural quasicrystals have bewildered and astonished scientists. Shechtman, the scientist who first worked with these peculiar structures and who was awarded the Nobel Prize for Chemistry in 2011, was ridiculed for his ideas and findings. Quasicrystals requires extremely precise conditions to form, including vacuum and an argon atmosphere. This has prompted many to claim these are improbable to find in nature. In 2007, however, Shechtman and geologist Luca Bindi from the University of Florence discovered strange quasicrystal-like formations inside a rock from Bindi’s collection. The rock in question was actually a meteorite, and following analysis indeed confirmed that the scientists were looking at quasicrystals. The researchers proved in 2012 that the crystals were formed in space by astrophysical phenomenonae, and not as a consequence of Earth collision or atmosphere entry. This demonstrated that quasicrystals could be found anywhere in the Universe.

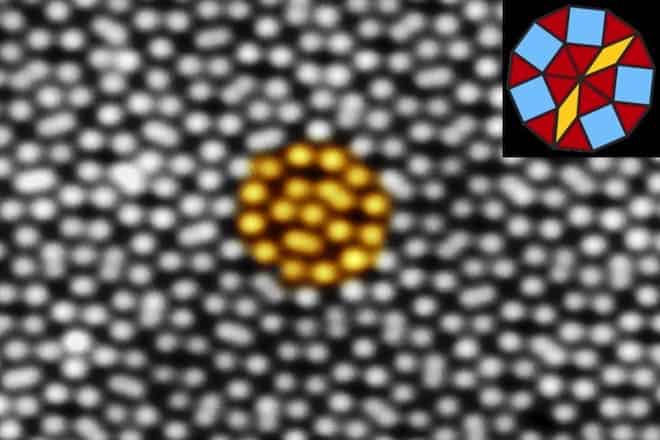
In Germany, the Martin Luther Uni scientists were studying how a certain kind of mineral called perovskite behaved when layered atop metallic platinum. The perovskite film was heated to high temperature. Upon inspection they found a 12-fold symmetry pattern – something that was thought to be impossible. Forster tried to resolve the 12-fold pattern, which clearly now was possible sitting right in front of them, into two groups with six-fold symmetry. It wasn’t possible. The only viable explanation was that they had created a thin, two-dimensional quasicrystalline layer.
“We were very surprised,” Widdra said. “It took quite a while until we were convinced that we had a new form of two-dimensional quasicrystal.”
The resulting quasicrystal is made out of 12-sided, dodecagonal arrangements with internal patterns of squares, triangles, and rhomboids. “They have a perfect order, but never repeat themselves,” Widdra said.
“This is another beautiful example of just how commonly quasicrystalline structures form,” said physicist Alan Goldman of Iowa State University and the U.S. Department of Energy’s Ames Laboratory, who was not involved in this study. “The number of examples continues to grow and continues to surprise us.”
The actual mechanics of quasicrystal formation are largely unknown so far. Why some materials can retain this structures and other can not is still a mystery.
“We really don’t understand why,” Goldman said. “Each new system provides us with some clues, and the more examples we find, the closer we come to answering that question.”
The 12-sided 2-D quasicrystal was described in a paper published in the journal Nature. [via Wired]